Learn about NIH's process for handling allegations of research misconduct, and what happens if findings are made.
Overview
NIH has specific procedures in place to handle allegations of research misconduct. All allegations received at the NIH are promptly and carefully reviewed. However, NIH does not have the authority to conduct investigations of research misconduct allegations except for those involving NIH intramural research. NIH conducts a preliminary assessment to determine whether allegations should be referred to the HHS Office of Research Integrity (ORI) for oversight. This assessment involves determining whether the allegation:
- Involves NIH-funded research or applications for NIH funds;
-
Is an allegation of research misconduct;
Whenever feasible, allegations that may be of concern to other federal agencies and/or NIH offices will be redirected as appropriate.
ORI is responsible for overseeing and directing PHS research integrity activities. ORI has the authority and the responsibility to review and monitor investigations of research misconduct allegations involving PHS funding.
NIH takes allegations of research misconduct seriously. Allegations are reviewed promptly and confidentially to protect the interests of the parties involved. This includes limiting information about the allegations to those with a need to know. NIH does not provide status updates on allegations or compliance reviews because of these privacy and confidentiality concerns.
Requirements for Making a Finding of Research Misconduct
The following provides the requirements for making a finding of research misconduct (per 42 CFR 93.104):
- Significant departure from accepted practices of the relevant research community
- Committed intentionally, knowingly, or recklessly
- Proven by a preponderance of evidence
Process for handling allegations of research misconduct received at NIH
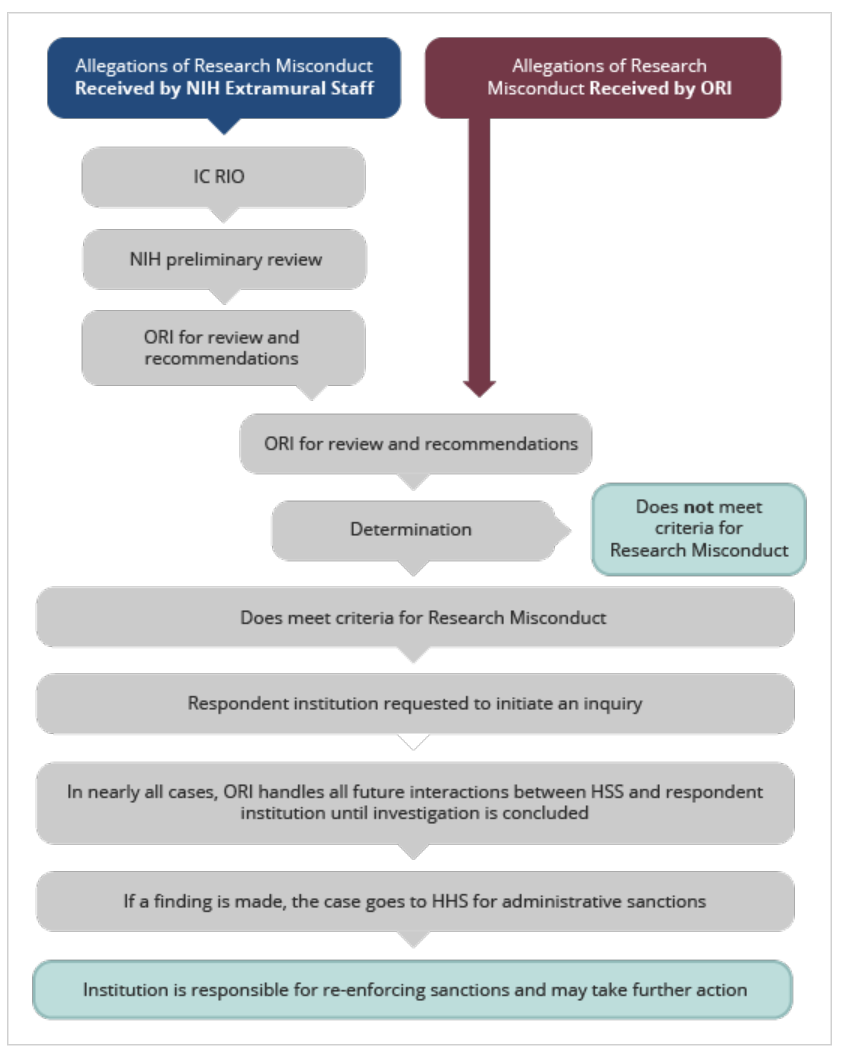
- An NIH Institute/Center (IC) extramural staff person forwards any allegation of research misconduct to their NIH Institute/Center (IC) Research Integrity Officer (RIO)
- The IC RIO forwards the allegation to the NIH Office of Extramural Research (OER) for handling.
- OER performs a preliminary review to assess for accuracy and sufficiency of the information provided, involvement of NIH applications/awards, and appropriateness for consideration as an allegation of research misconduct.
-
If appropriate, OER refers the allegation to ORI.
Allegations may also be referred to other offices, including the HHS Office of Human Research Protections (OHRP) for allegations involving human research participants, the NIH Office of Laboratory Animal Welfare (OLAW) for allegations involving animal welfare concerns, or the NIH Office of Management Assessment (OMA) for allegations involving the misuse of funds. -
ORI receives and assesses the allegation.
ORI will request the institution to initiate an inquiry if the allegation contains sufficient specific information, involves U.S. Public Health Service (PHS) funding, and fits the criteria for research misconduct. In many cases, ORI handles all interactions between HHS and the respondent institution until conclusion of the investigation. -
Alternatively, the allegation may be reported directly to ORI. In this
case, ORI performs the preliminary review.
(For details on the section that follows, please consult 42 CFR 93 , subpart B) -
The institution notifies the presumed respondent in writing and conducts
an inquiry to determine if the allegation warrants an investigation. An
investigation is warranted if there is
- A reasonable basis for concluding that the allegation falls within the definition of research misconduct and involves PHS supported research; and
- Preliminary information-gathering and fact-finding from the inquiry indicates that the allegation may have substance.
-
The inquiry must be completed within 60 calendar days of its initiation
unless circumstances clearly warrant a longer period.
The institution prepares a written report and provides the respondent an opportunity to review and comment on the inquiry report. The institution must notify the respondent whether the inquiry found that an investigation is warranted and may also notify the complainant who made the allegation. - Within 30 days of finding that an investigation is warranted, the institution must provide ORI with the written finding and a copy of the inquiry report.
-
The investigation must begin within 30 days after determining that an
investigation is warranted, and the ORI director and the respondent must
be notified.
The institution must take all reasonable and practical steps to obtain custody of all the research records and evidence needed to conduct the research misconduct proceeding before or at the time the institution notifies the respondent and whenever additional items become known or relevant to the investigation. An institution must complete all aspects of an investigation within 120 days of beginning it. -
The institution must give the respondent a copy of the draft
investigation report, and, concurrently, a copy of or supervised access
to, the evidence on which the report is based.
The respondent's copies on the draft report must be submitted within 30 days of the date the respondent received the draft report. The institution may also provide the complainant a copy of the draft report. - The institution provides ORI with:
- Investigation Report, including a copy of the report, all attachments, and any appeals.
- Final institutional action, stating whether the institution found research misconduct, and if so, who committed the misconduct.
- Whether the institution accepts the investigation's findings.
- Institutional administrative actions pending or completed against the respondent.
-
If a finding of research misconduct is made, the case goes to HHS for
administrative sanctions.
The Assistant Secretary for Health (ASH) makes the final PHS/HHS decision on the imposition of administrative actions after reviewing the recommendations made by ORI, except when the administrative actions include debarment or suspension. The ASH may accept, modify, or reject the administrative actions recommended by ORI. -
The institution is responsible for enforcing sanctions.
The institution and NIH may add additional sanctions.