If John is a paid employee of Science University, which has submitted a research application to NIH, can he serve on the panel that reviews the application? Since John has a conflict of interest with the application, he may not review that particular application and will have to be out of the room during the discussion and evaluation of that application, but may serve on the panel to review other applications.
The core values of impartiality, fairness, and integrity are fundamental to the NIH peer review process (PDF, 774 KB). NIH Scientific Review Officers (SROs) spend considerable time and energy identifying appropriate reviewers and managing reviewer conflicts of interest (COI).
Application of the Rules
The rules for managing COI addressed on this page apply to peer reviewers participating in:
- initial peer review for all types of grant programs, with the exception of construction grants, and
- peer review of proposals for Research and Development (R & D) contracts.
When does COI arise?
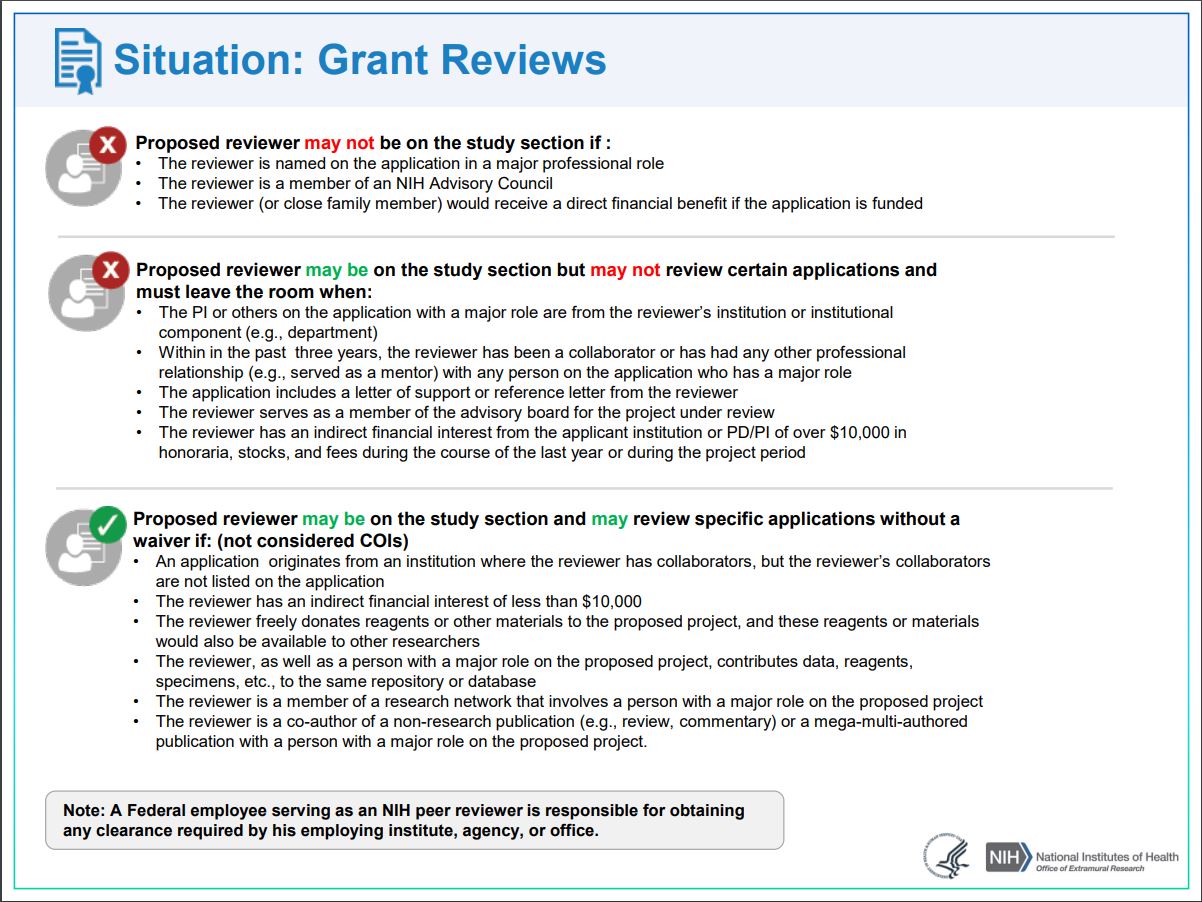
What are the types of conflicts that must be managed? Check out the many types below. Note that COI is handled differently for reviewers of grants and R&D contracts.
Grants Reviews
Contract Reviews
- Appearance of a COI: Any situation that could cause a reasonable person with all the relevant facts to question the impartiality of the reviewer or that leads a reviewer to question his or her objectivity means that the reviewer:
- May not participate in the evaluation of that grant application.
- May not serve on the study section where that R&D contract proposal is evaluated.
- Study section membership: An application from a member of a study section that meets regularly may not be reviewed by that member's study section.
- Applications in response to a Request for Applications (RFA): An individual who is listed on an application submitted to an RFA with a major professional role may not serve on a study section evaluating any applications from that same RFA.
Certifying COI
Each NIH peer reviewer must certify, under penalty of perjury (US Code Title 18 chapter 47 section 1001), that to the best of his or her knowledge he/she has disclosed all conflicts of interest that he or she may have with the applications or R&D contract proposals; he or she fully understands the confidential nature of the review process and agrees:
- to destroy or return all materials related to it;
- not to disclose or discuss the materials associated with the review, the evaluation, or the review meeting with any other individual except as authorized by the Scientific Review Officer (SRO) or other designated NIH official;
- not to disclose procurement information prior to the award of a contract; and
- to refer all inquiries concerning the review to the SRO or other designated NIH official.
Want more policy details?
- NOT-OD-21-005: Notice of Policy Revision: Managing Conflicts of Interest for Requests for Applications (RFAs)
- NOT-OD-21-019: Notice of Clarification: Policy for Managing Conflict of Interest in NIH Peer Review
- NOT-OD-14-069: NIH Policy for Managing Conflict of Interest in the Peer Review of Concepts and Proposals for R&D Contract Projects
- NOT-OD-13-010: Advance Notice: Revised Policy for Managing Conflict of Interest in the Initial Peer Review of NIH Grant and Cooperative Agreement Applications
- NIH Conflict of Interest Rules for Reviewers (PDF, 34 KB)