NIH has launched a series of initiatives to enhance the accountability and transparency of clinical research. These initiatives target key points along the clinical trial lifecycle from concept to results reporting. Find key resources and tips for applicants and grantees.
Your human subjects study may meet
the NIH definition of a clinical trial.
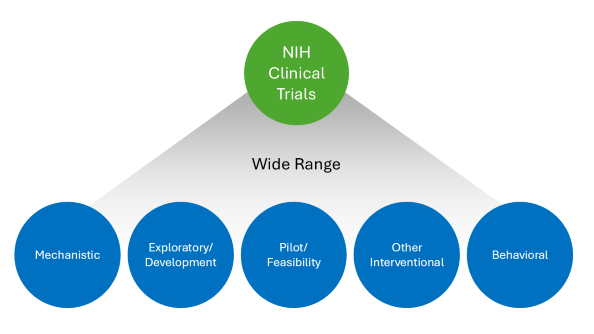

NIH's Definition of a Clinical Trial
Learn more about why NIH has implemented changes to enhance the stewardship of clinical trials and how these changes impact your research.

Is My Project a Clinical Trial, Basic Experimental Study Involving Humans (BESH), or an Observational Study Involving Humans?
Learn how to determine whether your project is a clinical trial, basic experimental study involving humans (BESH), or an observational study involving humans. Check what to consider when answering the four clinical trial questions, case studies, and tips for BESH investigators.

Clinical Trial-Specific Funding Opportunities
All applicants proposing clinical trials can learn about the requirement for submitting applications through a funding opportunity designated specifically for clinical trials.

Clinical Trial-Specific Review Criteria
Specific review criteria will be used to evaluate applications proposing clinical trials or clinical trial research experience. Learn more about these review criteria.

Good Clinical Practice
All NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials can learn about the requirement to be trained in Good Clinical Practice (GCP).
Human Subjects System
Effective June 9, 2018, the Human Subjects System (HSS) replaced the Inclusion Management System (IMS). HSS consolidates human subjects and clinical trial information in one place. PD/PIs, signing officials, and NIH staff access the HSS through eRA Commons.

Human Subjects and Clinical Trial Information Form
The Human Subjects and Clinical Trial Information form is required for all human subjects and/or clinical trial research beginning with the January 25, 2018 due dates. Learn more about this form and tips for completing it.

Posting Clinical Trial Informed Consent Forms
Learn more about the requirement that clinical trials post informed consent documents to a public federal government website.

Protocol Templates for Clinical Trials
Discover our protocol templates with instructional and sample text to help write clinical protocols for the following types of research: 1) Phase 2 or 3 clinical trials that require Investigational New Drug applications (IND) or Investigational Device Exemption (IDE) applications, and 2) Behavioral and social sciences research involving humans.

Requirements for Registering & Reporting NIH-Funded Clinical Trials
All NIH-funded clinical trials are expected to register and submit results information to ClinicalTrials.gov. Learn more about these requirements. Learn more about what you need to know about these requirements.

Information about NIH Clinical Trial Stewardship
Learn why clinical trial policies are key to enhancing the stewardship of clinical trials.

Training & Resources for NIH Clinical Trial Policies
Learn about training opportunities, find helpful resources, and use the links below for guidance on NIH's clinical trial policies. We will continue to post additional resources, so check back frequently.