Revised: December 7, 2018
G.400 - PHS 398 Research Plan Form
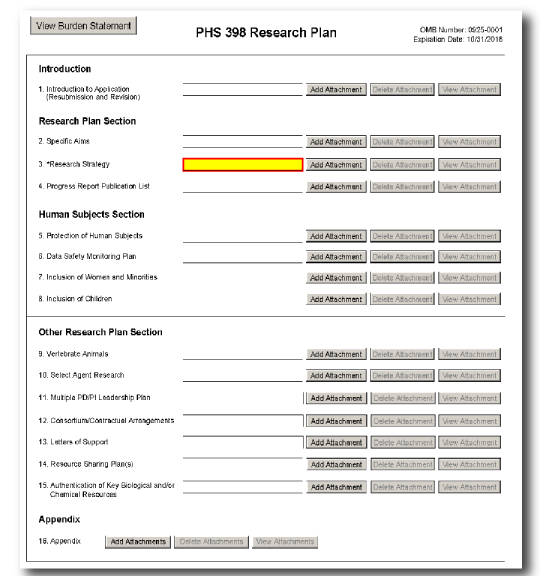
The PHS 398 Research Plan form is used only for Research, Multi-project, and SBIR/STTR applications.
This form includes fields to upload several attachments, including the Specific Aims and Research Strategy.
The Research Plan should include sufficient information needed for evaluation of the project, independent of any other document (e.g., previous application). Be specific and informative, and avoid redundancies.
| # | Description |
|---|---|
| - | Introduction |
| - | Research Plan Section |
| - | Human Subjects Section |
| - | Other Research Plan Section |
| - | Appendix |
Your application should represent a sound approach to the investigation of an important biomedical research, behavioral research, technological, engineering, or scientific question, and be worthy of support under the stated criteria of the FOA. It should be self-contained and written with the care and thoroughness accorded to papers for publication.
Review the application carefully to ensure you have included information essential for evaluation. The scientific and technical merit of the proposed research is the primary concern for all research supported by the National Institutes of Health (NIH) and other PHS agencies.
Read all the instructions in the FOA before completing this section to ensure that your application meets all IC-specific criteria.
Who should use the PHS 398 Research Plan Form:
Use the PHS 398 Research Plan Form only if you are submitting a Research, Multi-project, or SBIR/STTR application.
Additional Instructions for SBIR/STTR:
You are strongly encouraged to contact agency program staff for pre-application guidance and/or for more specific information on the research topics described in the solicitation.
The applicant small business must not propose market research, patent applications, or litigation. The research proposed in this application may, however, be carried out through construction and evaluation of a laboratory prototype, where necessary.
Note to all Commercialization Readiness Pilot (CRP) Program Applications:
CRP uses SBIR funding, but is not a Phase I/II/IIB or Fast-Track application. However, CRP Applications should follow all Phase II-specific instructions.
 Applicants must follow all policies and requirements related to proprietary information, page limits and formatting. See the following pages for more information:
Applicants must follow all policies and requirements related to proprietary information, page limits and formatting. See the following pages for more information:
- NIH's Formatting Attachments
- NIH's Table of Page Limits
- Proprietary Information: Section 2.3.11.2: Confidentiality of Information and Section 2.3.11.2.2: The Freedom of Information Act of the NIH Grants Policy Statement
Introduction
1. Introduction to Application (Resubmission and Revision)
Who must complete the "Introduction to Application" section:
An "Introduction to Application" attachment is required only if the Type of Application is Resubmission or Revision or if the FOA specifies that one is needed. An Introduction is not allowed for New or Renewal applications.
Descriptions of different types of applications are listed here: NIH Types of Applications.
Format:
Follow the page limits for the Introduction in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Resubmission applications: See specific instructions on the content of the Introduction on the NIH Resubmission Applications website.
Competing Revisions: See specific instructions on the content of the Introduction on the NIH Competing Revisions website.
Additional Instructions for Multi-project:
Overall Component: The "Introduction" attachment is required for all Resubmission and Revision applications.
Other Components: The "Introduction" attachment is optional for Resubmissions and Revisions applications. Although the "Introduction" attachment is optional, you may get a system warning if there is no attachment.
Research Plan Section
2. Specific Aims
Who must complete the "Specific Aims" section:
The "Specific Aims" attachment is required unless otherwise specified in the FOA.
Format:
Follow the page limits for the Specific Aims in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
State concisely the goals of the proposed research and summarize the expected outcome(s), including the impact that the results of the proposed research will have on the research field(s) involved.
List succinctly the specific objectives of the research proposed, e.g., to test a stated hypothesis, create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice, address a critical barrier to progress in the field, or develop new technology.
Additional Instructions for Multi-project:
Overall Component: The "Specific Aims" attachment is required.
Other Components: The "Specific Aims" attachment is required.
Additional Instructions for SBIR/STTR:
Phase I Applications: State the specific objectives of the Phase I research and development effort, including the technical questions you will try to answer to determine the Phase I feasibility of the proposed approach and the impact that the results of the proposed research will exert on the research field(s) involved. State concisely and realistically what the proposed research is intended to accomplish in terms of its potential for technological innovation and commercial application. Define the proposed product, process or service to ultimately be developed. Include milestones for each of the aims as these will be used in the evaluation process.
Phase II, Phase IIB, and CRP Applications: State the specific objectives of the Phase II research and development effort including the impact that the results of the proposed research will exert on the research field(s). State concisely and realistically what the proposed research is intended to accomplish in terms of its potential for technological innovation and commercial application. Define the proposed product, process or service to ultimately be developed. Include milestones for each of the aims as these will be used in the evaluation process.
Fast-Track Applications: Create a heading titled "Phase I Specific Aims", and follow the instructions above for "Phase I Applications." Next, create a heading titled "Phase II Specific Aims" and follow the instructions above for "Phase II Applications." Note that the page limit applies to both phases in combination, not to each phase individually.
3. Research Strategy
Who must complete the "Research Strategy" section:
The "Research Strategy" attachment is required.
Format:
Follow the page limits for the Research Strategy in the NIH Table of Page Limits, unless otherwise specified in the FOA. Although multiple sections of information are required in the Research Strategy as detailed below, the page limit applies to the entirety of the single Research Strategy attachment.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Organize the Research Strategy in the specified order and using the instructions provided below unless otherwise specified in the FOA. Start each section with the appropriate heading - Significance, Innovation, Approach.
Cite published experimental details in the Research Strategy section and provide the full reference in G.220 - R&R Other Project Information Form, Bibliography and Reference Cited.
 1. Significance
1. Significance
- Explain the importance of the problem or critical barrier to progress that the proposed project addresses.
- Describe the scientific premise for the proposed project, including consideration of the strengths and weaknesses of published research or preliminary data crucial to the support of your application.
- Explain how the proposed project will improve scientific knowledge, technical capability, and/or clinical practice in one or more broad fields.
Additional Instructions for Research:
Describe how the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field will be changed if the proposed aims are achieved.
Additional Instructions for Multi-project:
Overall and Other Components: Describe how the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field will be changed if the proposed aims are achieved.
Additional Instructions for SBIR/STTR:
Explain the project's potential to lead to a marketable product, process, or service.
Phase II, CRP, Fast-Track, and Phase IIB Competing Renewals: Explain how the commercialization plan demonstrates a high probability of commercialization.
2. Innovation
- Explain how the application challenges and seeks to shift current research or clinical practice paradigms.
- Describe any novel theoretical concepts, approaches or methodologies, instrumentation or interventions to be developed or used, and any advantage over existing methodologies, instrumentation, or interventions.
- Explain any refinements, improvements, or new applications of theoretical concepts, approaches or methodologies, instrumentation, or interventions.
3. Approach
- Describe the overall strategy, methodology, and analyses to be used to accomplish the specific aims of the project. Describe the experimental design and methods proposed and how they will achieve robust and unbiased results. Unless addressed separately in the Resource Sharing Plan, include how the data will be collected, analyzed, and interpreted, as well as any resource sharing plans as appropriate.
- Discuss potential problems, alternative strategies, and benchmarks for success anticipated to achieve the aims.
- If the project is in the early stages of development, describe any strategy to establish feasibility, and address the management of any high risk aspects of the proposed work.
- Explain how relevant biological variables, such as sex, are factored into research designs and analyses for studies in vertebrate animals and humans. For example, strong justification from the scientific literature, preliminary data, or other relevant considerations, must be provided for applications proposing to study only one sex.
- Please refer to NIH Guide Notice on Sex as a Biological Variable in NIH-funded Research for further consideration of NIH expectations about sex as a biological variable.
- If your study(s) involves human subjects, the sections on the Question 7: Inclusion of Women and Minorities and Question 8: Inclusion of Children can be used to expand your discussion on inclusion and justify the proposed proportions of individuals (such as males and females) in the sample, but it must also be addressed here in the "Approach" section of the "Research Strategy" attachment.
- Point out any procedures, situations, or materials that may be hazardous to personnel and the precautions to be exercised. A full discussion on the use of select agents should appear in Question 10: Select Agent Research, below.
- If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line from the NIH hESC Registry cannot be chosen, provide a strong justification for why an appropriate cell line cannot be chosen from the Registry at this time.
Additional Instructions for SBIR/STTR:
Provide a tentative sequence or timetable for the project.
If you have multiple Specific Aims, you may address the Significance, Innovation, and Approach either for each Specific Aim individually or for all of the Specific Aims collectively.
As applicable, also include the following information as part of the Research Strategy, keeping within the three sections (Significance, Innovation, and Approach) listed above.
Preliminary Studies for New Applications:
For New Applications, include information on Preliminary Studies. Discuss the PD/PI's preliminary studies, data, and or experience pertinent to this application. Except for Exploratory/Developmental Grants (R21/R33), Small Research Grants (R03), and Academic Research Enhancement Award (AREA) Grants (R15), preliminary data can be an essential part of a research grant application and can help to establish the likelihood of success of the proposed project. Early Stage Investigators should include preliminary data.
Additional Instructions for SBIR/STTR:
Phase I Applications: Preliminary data are not required for Phase I Applications; however, such results may assist reviewers in assessing the likelihood of success of the proposed project and may be included in the Research Strategy section.
Progress Report for Renewal and Revision Applications:
Note that the Progress Report falls within the Research Strategy and is therefore included in the page limits for the Research Strategy.
For Renewal/Revision Applications, provide a Progress Report. Provide the beginning and ending dates for the period covered since the last competitive review. In the Progress Report, you should:
- Summarize the specific aims of the previous project period and the importance of the findings, and emphasize the progress made toward their achievement.
- Explain any significant changes to the specific aims and any new directions, including changes resulting from significant budget reductions.
- Discuss previous participant enrollment (e.g., recruitment, retention, inclusion of women, minorities, children, etc.) for any studies meeting the NIH definition for clinical research, particularly if relevant to studies proposed in the Renewal or Revision Application. You should not submit a PHS Inclusion Enrollment Report Form unless the enrollment is part of new or ongoing studies in the Renewal or Revision Application.
Do not include a list of publications, patents, or other printed materials in the Progress Report. That information will be included in the "Progress Report Publication List" attachment.
Additional Instructions for SBIR/STTR:
Phase II, Phase IIB, and CRP Competing Renewal and Revision Applications: In the Progress Report, in addition to what's listed above, describe the technology developed from this SBIR/STTR, its intended use, and who will use it. Describe the current status of the product (e.g., under development, commercialized, in use, discontinued). If applicable, describe the status of FDA approval for your product, process, or service (e.g., continuing pre-IND studies, filed on IND, in Phase I (or II or III) clinical trials, applied for approval, review ongoing, approved, not approved).
4. Progress Report Publication List
Who must complete the "Progress Report Publication List" section:
A "Progress Report Publication List" attachment is required only if the Type of Application is Renewal.
Descriptions of different types of applications are listed here: NIH's Types of Applications.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
List the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, and other printed materials that have resulted from the project since it was last reviewed competitively.
Provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for each of the following:
- Articles that fall under the Public Access Policy,
- Articles that were authored or co-authored by the applicant and arose from NIH support,
- Articles that were authored or co-authored by the applicant and arose from AHRQ funding provided after 2/19/16 (see Guide Notice on Policy for Public Access to AHRQ-Funded Scientific Publications).
If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate "PMC Journal - In Process." NIH maintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Additional Instructions for Multi-project:
Overall and Other Components: If you include a "Progress Report Publication List" attachment, you can include it in either the Overall Component or within each Other Component, but do not attach the same information in multiple locations.
Additional Instructions for SBIR/STTR:
Phase II, Phase IIB, and CRP Applications: List the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, copyrights, trademarks, invention reports and other printed materials, if any, that resulted from the Phase I or describe patent status, trade secrets or other demonstration of IP protection, and other printed materials that have resulted from the Phase I effort.
Human Subjects Section
5. Protection of Human Subjects
Who must complete the "Protection of Human Subjects" section:
Include a "Protection of Human Subjects" attachment if you answered "Yes" to the question "Are human subjects involved?" on the G.220 - R&R Other Project Information Form.
If you answered "No" to the "Are human subjects involved" question but your proposed research involves human specimens and/or data from subjects, you must provide a justification in this section for your claim that no human subjects are involved.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Do not use the "Protection of Human Subjects" section to circumvent the page limits of the Research Strategy.
Content:
Refer to
For more information:
Refer to the NIH's Research Involving Human Subjects site.
Additional Instructions for Multi-project:
Overall Component: The "Protection of Human Subjects" attachment is optional unless specifically requested in the FOA.
Other Components: The "Protection of Human Subjects" attachment is required if you answered "Yes" to the question "Are human subjects involved?" on the Section G.220 - R&R Other Project Information Form.
6. Data Safety Monitoring Plan
Who must complete the "Data Safety Monitoring Plan" section:
Include a "Data Safety Monitoring Plan" attachment if you answered "Yes" to the question "Clinical Trial?" on the PHS 398 Cover Page Supplemental Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Refer to
Additional Instructions for Multi-project:
Overall Component: The "Data Safety Monitoring Plan" attachment is optional unless specifically requested in the FOA.
Other Components: The "Data Safety Monitoring Plan" is required if you answered "Yes" to the question "Clinical Trial?" on the PHS Cover Page Supplemental Form.
7. Inclusion of Women and Minorities
Who must complete the "Inclusion of Women and Minorities" section:
Include an "Inclusion of Women and Minorities" attachment if you answered "Yes" to the question "Are human subjects involved?" on the R&R Other Project Information Form and the research does not fall under Exemption 4.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Refer to
Additionally, refer to G.500 - PHS Inclusion Enrollment Report as well as the
Additional Instructions for Multi-project:
Overall Component: The "Inclusion of Women and Minorities" attachment is optional unless specifically requested in the FOA.
Other Components: The "Inclusion of Women and Minorities" is required if you answered "Yes" to the question "Are human subjects involved?" on the R&R Other Project Information Form and the research does not fall under Exemption 4.
8. Inclusion of Children
Who must complete the "Inclusion of Children" section:
Include an "Inclusion of Children" attachment if you answered "Yes" to the question "Are human subjects involved?" on the R&R Other Project Information Form and the research does not fall under Exemption 4.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Refer to the
Additional Instructions for Multi-project:
Overall Component: The "Inclusion of Children" attachment is optional unless specifically requested in the FOA.
Other Components: The "Inclusion of Children" is required if you answered "Yes" to the question "Are human subjects involved?" on the R&R Other Project Information Form and the research does not fall under Exemption 4.
Other Research Plan Section
9. Vertebrate Animals
Who must complete the "Vertebrate Animals" section:
Include a "Vertebrate Animals" attachment if you answered "Yes" to the question "Are Vertebrate Animals Used?" on the R&R Other Project Information Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Do not use the Vertebrate Animals section to circumvent the page limits of the Research Strategy.
Content:
If vertebrate animals are involved in the project, address each of the following criteria:
- Description of Procedures: Provide a concise description of the proposed procedures to be used that involve vertebrate animals in the work outlined in the "Research Strategy" section. Identify the species, strains, ages, sex, and total numbers of animals by species, to be used in the proposed work. If dogs or cats are proposed, provide the source of the animals.
- Justifications: Provide justification that the species are appropriate for the proposed research. Explain why the research goals cannot be accomplished using an alternative model (e.g. computational, human, invertebrate, in vitro).
- Minimization of Pain and Distress: Describe the interventions including analgesia, anesthesia, sedation, palliative care and humane endpoints that will be used to minimize discomfort, distress, pain, and injury.
Provide a concise, complete description of the animals and proposed procedures. In addition to the 3 points above, you should also:
- Identify all project/performance or collaborating site(s) and describe activities of proposed research with vertebrate animals in those sites.
- Explain when and how animals are expected to be used if plans for the use of animals have not been finalized.
For more information:
See the NIH's Office of Laboratory Animal Welfare website.
See NIH's Vertebrate Animals Section Worksheet.
An applicable Animal Welfare Assurance will be required if the grantee institution does not have one (see
Additional Instructions for Multi-project:
Overall Component: The "Vertebrate Animals" attachment is optional unless specifically requested in the FOA.
Other Components: Complete the "Vertebrate Animals" section if you answered "Yes" to the question "Are Vertebrate Animals Used?" on the R&R Other Project Information Form.
10. Select Agent Research
Who must complete the "Select Agent Research" section:
Include a "Select Agent Research" attachment if your proposed activities involve the use of select agents at any time during the proposed project period, either at the applicant organization or at any performance site.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
For more information:
Select agents are hazardous biological agents and toxins that have been identified by HHS or the U.S. Department of Agriculture (USDA) as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. The Centers for Disease control and Prevention (CDC) and the Animal APHIS Select Agent Programs jointly maintain a list of these agents. See the Federal Select Agent Program website.
See also the Supplemental Instructions, Part III, Section 2.13: Select Agent Research.
Content:
Excluded select agents: If the activities proposed in the application involve only the use of a strain(s) of select agents which has been excluded from the list of select agents and toxins as per 42 CFR 73.3, the select agent requirements do not apply. Use this "Select Agent Research" section to identify the strain(s) of the select agent that will be used and note that it has been excluded from this list. The CDC maintains a list of exclusions, which is available on the Select Agents and Toxins Exclusions website.
Applying for a select agent to be excluded: If the strain(s) is not currently excluded from the list of select agents and toxins but you have applied or intend to apply to HHS for an exclusion from the list, use this section to indicate the status of your request or your intent to apply for an exclusion and provide a brief justification for the exclusion.
All applicants proposing to use select agents: Address the following three points for each site at which select agent research will take place. Although no specific page limitation applies to this section, be succinct.
- Identify the select agent(s) to be used in the proposed research.
- Provide the registration status of all entities* where select agent(s) will be used.
- If the performance site(s) is a foreign institution, provide the name(s) of the country or countries where select agent research will be performed.
- *An "entity" is defined in 42 CFR 73.1 as "any government agency (Federal, State, or local), academic institution, corporation, company, partnership, society, association, firm, sole proprietorship, or other legal entity."
- Provide a description of all facilities where the select agent(s) will be used.
- Describe the procedures that will be used to monitor possession, use, and transfer of select agent(s).
- Describe plans for appropriate biosafety, biocontainment, and security of the select agent(s).
- Describe the biocontainment resources available at all performance sites.
11. Multiple PD/PI Leadership Plan
Who must complete the "Multiple PD/PI Leadership Plan" section:
Any applicant who designates multiple PD/PIs (on the R&R Senior/Key Person Profile (Expanded) Form) must include a Multiple PD/PI Leadership Plan. For applications designating multiple PD/PIs, all such individuals must be assigned the PD/PI role on the R&R Senior/Key Profile (Expanded) Form, even those at organizations other than the applicant organization.
Do not submit a Multiple PD/PI Leadership Plan if you are not submitting a Multiple PD/PI Application.
Additional Instructions for Multi-project:
Overall Component: The "Multiple PD/PI Leadership Plan" attachment is required if more than one PD/PI is specified on the Overall Component's R&R Senior/Key Person Profile Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
A rationale for choosing a multiple PD/PI approach should be described. The governance and organizational structure of the leadership team and the research project should be described, including communication plans, processes for making decisions on scientific direction, and procedures for resolving conflicts. The roles and administrative, technical, and scientific responsibilities for the project or program should be delineated for the PD/PIs and other collaborators.
If budget allocation is planned, the distribution of resources to specific components of the project or the individual PD/PIs should be delineated in the Multiple PD/PI Leadership Plan. In the event of an award, the requested allocations may be reflected in a footnote on the Notice of Grant Award.
For more information:
For background information on the Multi-PD/PI initiative, see NIH's Multiple Principal Investigators page.
12. Consortium/Contractual Arrangements
Who must complete the "Consortium/Contractual Arrangements" section:
Include a "Consortium/Contractual Arrangements" attachment if you have consortiums/contracts in your budget.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Explain the programmatic, fiscal, and administrative arrangements to be made between the applicant organization and the consortium organization(s). If consortium/contractual activities represent a significant portion of the overall project, explain why the applicant organization, rather than the ultimate performer of the activities, should be the grantee.
Note: The signature of the Authorized Organization Representative in G.200 - SF 424 (R&R), Question 19: Authorized Representative signifies that the applicant and all proposed consortium participants understand and agree to the following statement:
The appropriate programmatic and administrative personnel of each organization involved in this grant application are aware of the agency's consortium agreement policy and are prepared to establish the necessary inter-organizational agreement(s) consistent with that policy.
For more information:
Please refer to the NIH Grants Policy Statement, Section 15: Consortium Agreements for more information.
Additional Instructions for Multi-project:
Overall and Other Components: Unless otherwise specified in the FOA, you have the option to:
- include a single consolidated "Consortium/Contractual Arrangements" attachment in the Overall Component, or
- include component-specific "Consortium/Contractual Arrangements" attachment(s) within the components that include subawards, or
- include a "Consortium/Contractual Arrangements" attachment in both the Overall Component and Other Component(s).
Do not include the same attachment in multiple locations.
Additional Instructions for SBIR/STTR:
SBIR:
Phase I Applications: Normally, a minimum of two-thirds or 67% of the research or analytical effort must be carried out by the small business concern (SBC). The total amount of all consultant and contractual arrangements to third parties for portions of the scientific and technical effort generally may not exceed 33% of the total amount requested (direct, F&A/indirect, and fee).
Phase II and Phase IIB Applications: Normally, a minimum of one-half or 50% of the research or analytical effort must be carried out by the SBC. The total amount of consultant and contractual arrangements to third parties for portions of the scientific and technical effort generally may not exceed 50% of the total Phase II amount requested (direct, F&A/indirect, and fee).
Phase I and Phase II Applications: The basis for determining the percentage of work to be performed by each of the cooperative parties in Phase I or Phase II will be the total requested costs (direct, F&A/indirect, and fee) attributable to each party, unless otherwise described and justified in G.400 - PHS 398 Research Plan Form, Consortium/Contractual Arrangements.
Fast-Track SBIR Applications: Create two separate sections entitled "Phase I Consortium/Contractual Arrangements" and "Phase II Consortium/Contractual Arrangements", and complete the sections following the instructions provided above for each phase.
Fast-Track SBIR Applications: Create two separate sections entitled "Phase I Consortium/Contractual Arrangements" and "Phase II Consortium/Contractual Arrangements", and complete the sections following the instructions provided above for each phase.
STTR:
Phase I, Phase II and Phase IIB STTR Applications: At least 40% of the work must be performed by the SBC and at least 30% of the work must be performed by the single partnering research institution. The basis for determining the percentage of work to be performed by each of the cooperative parties will be the total of the requested costs (direct, F&A/indirect, and fee) attributable to each party, unless otherwise described and justified in G.400 - PHS 398 Research Plan Form, Consortium/Contractual Arrangements.
Certification showing the cooperative R&D arrangement between the SBC and the research institution will be requested prior to an award.
The single partnering research institution must certify at the time of application that at least 30% of the work of the STTR project will be performed by the research institution. This 30% requirement applies to the single collaborating organization identified as the "research institution."
The requisite signature, printed name, title, and date of signature of the duly authorized representative of the research institution affirming certifications made by the research institution must be included in a letter stating:
"The small business concern and the research institution certify jointly that: (1) the proposed STTR project will be conducted jointly by the small business concern and the research institution in which not less than 40 percent of the work will be performed by the small business concern and not less than 30 percent of the work will be performed by the research institution ("cooperative research and development"); (2) the proposed STTR project is a cooperative research or research and development effort to be conducted jointly by the small business concern and the research institution in which not less than 40 percent of the work will be performed by the small business concern and not less than 30 percent of the work will be performed by the research institution ("performance of research and analytical work"); and (3) regardless of the proportion of the proposed project to be performed by each party, the small business concern will be the primary party that will exercise management direction and control of the performance of the project.
If the research institution is a contractor-operated Federally Funded Research and Development Center (FFRDC), the duly authorized representative of the contractor-operated Federally funded research and development center certifies, additionally, that it: "(4) is free from organizational conflicts of interests relative to the STTR program; (5) did not use privileged information gained through work performed for an STTR agency or private access to STTR agency personnel in the development of this STTR grant application; and (6) used outside peer review, as appropriate, to evaluate the proposed project and its performance therein."
The applicant SBC should convert the letter from the partnering research institution into a PDF attachment, and include it as part of this section (G.400 - PHS 398 Research Plan Form, Consortium/Contractual Arrangements).
Fast-Track STTR Applications: Create two separate sections entitled "Phase I Consortium/Contractual Arrangements" and "Phase II Consortium/Contractual Arrangements", and complete the sections following the instructions provided above for each phase.
13. Letters of Support
Format:
Combine all letters of support into a single PDF file and attach this information here. Do not place these letters in the Appendix. Follow the attachment guidelines on NIH's Format Attachments page.
Content:
Attach a file with all letters of support, including any letters necessary to demonstrate the support of consortium participants and collaborators such as Senior/Key Personnel and Other Significant Contributors included in the grant application.
Letters should stipulate expectations for co-authorship, and whether cell lines, samples, or other resources promised in the letter are freely available to other investigators in the scientific community or will be provided to the particular investigators only.
For consultants, letters should include rate/charge for consulting services and level of effort/number of hours per budget period anticipated. In addition, letters ensuring access to core facilities and resources should stipulate whether access will be provided as a fee-for-service.
Letters are not required for personnel (such as research assistants) not contributing in a substantive, measurable way to the scientific development or execution of the project.
Do not include consultant biographical sketches in the "Letters of Support" section, as consultant biosketches should be in the "Biographical Sketch" section (
Additional Instructions for SBIR/STTR:
Involvement of consultants and collaborators in the planning and research stages of the project is permitted. With the application, include letters from each individual and/or collaborator confirming their role(s) in the project. The letter(s) should be prepared on the consultant or collaborator's letterhead and addressed to the SBC. One page is recommended.
At a minimum, each consultant and collaborator letter should (1) verify their commitment to the project; (2) refer to the specific project by name, acknowledging the PD/PI as the lead on the project; and (3) specify what services /tasks the consultant or collaborator will contribute (e.g. expertise, number of hours/ percent of effort, summary of tasks to be completed). For consultants, the letter should also include the rate/charge for consulting services. Also include biographical sketches for each consultant.
Letters of interest from potential commercial partners or investors and letters of commitment of funds or other resources that will enhance the likelihood of commercialization should be placed following the letters of support for consultants and collaborators.
STTR only: The single "partnering" research institution must provide a letter to the applicant small business concern certifying that at least 30% of the work of the STTR project will be performed by the research institution.
14. Resource Sharing Plan(s)
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Data Sharing Plan: Investigators seeking $500,000 or more in direct costs (exclusive of consortium F&A) in any budget period are expected to include a brief 1-paragraph description of how final research data will be shared, or explain why data-sharing is not possible (for example human subject concerns, the Small Business Innovation Development Act provisions, etc.). Specific FOAs may require that all applications include this information regardless of the dollar level. Applicants are encouraged to read the FOA carefully and discuss their data-sharing plan with their program contact at the time they negotiate an agreement with the Institute/Center (IC) staff to accept assignment of their application. For more information, see the NIH Data Sharing Policy or the NIH Guide Notice on Sharing Research Data.
Sharing Model Organisms: Regardless of the amount requested, all applications where the development of model organisms is anticipated are expected to include a description of a specific plan for sharing and distributing unique model organisms or state why such sharing is restricted or not possible. For more information, see
Genomic Data Sharing (GDS): Applicants seeking funding for research that generates large-scale human or non-human genomic data are expected to provide a plan for sharing of these data. Examples of large-scale genomic data include genome-wide association studies (GWAS), single nucleotide polymorphisms (SNP) arrays, and genome sequence, transcriptomic, epigenomic, and gene expression data. Supplemental Information to the NIH GDS provides examples of genomic research projects that are subject to the Policy. For more information see the NIH GDS Policy, the NIH Guide Notice on Genomic Data Sharing Policy, and the GDS website.
Note on GDS: For proposed studies generating human genomic data under the scope of the GDS Policy, an Institutional Certification may be submitted at the time of application submission, but it is not required at that time. The Institutional Certification, however, will be requested as Just-in-Time (JIT) information prior to award. The Institutional Certification, or in some cases, a Provisional Institutional Certification, must be submitted and accepted before the award can be issued.
For more information:
NIH considers the sharing of unique research resources developed through NIH-sponsored research an important means to enhance the value and further the advancement of the research. When resources have been developed with NIH funds, and the associated research findings published or provided to NIH, it is important that they be made readily available for research purposes to qualified individuals within the scientific community. See
15. Authentication of Key Biological and/or Chemical Resources
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
If applicable to the proposed science, briefly describe methods to ensure the identity and validity of key biological and/or chemical resources used in the proposed studies. A maximum of one page is suggested.
For more Information:
Key biological and/or chemical resources are characterized as follows.
- Key biological and/or chemical resources may or may not be generated with NIH funds and: 1) may differ from laboratory to laboratory or over time; 2) may have qualities and/or qualifications that could influence the research data; and 3) are integral to the proposed research. These include, but are not limited to, cell lines, specialty chemicals, antibodies, and other biologics.
- Standard laboratory reagents that are not expected to vary do not need to be included in the plan. Examples are buffers and other common biologicals or chemicals.
- See NIH's page on Rigor and Reproducibility for more information.
Appendix
16. Appendix
Refer to the FOA to determine whether an Appendix is allowed in your application.
Note: The Appendix policy will be changing as of January 24, 2017. Please note that there are two sets of instructions below, based on the application due dates.
For applications submitted for due dates on or before January 24, 2017:
Format:
See NIH's Format Attachments page. A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 Appendix attachments are needed, combine the remaining information into attachment #10. Note that this is the total number of Appendix items, not the total number of publications.
For materials that cannot be submitted electronically or materials that cannot be converted to PDF (e.g., medical devices, prototypes, DVDs, CDs), applicants should contact the Scientific Review Officer following notification of assignment of the application to a study section. Applicants are encouraged to be as concise as possible and submit only information essential for the review of the application.
Do not use the Appendix to circumvent the page limits of the Research Strategy or of any other section of the application for which a page limit applies. For additional information regarding Appendix material and page limits, please refer to the NIH Guide Notice on Compliance with NIH Application Format and Content Instructions.
Use filenames for attachments that are descriptive of the content.
A summary sheet listing all of the items included in the Appendix is encouraged but not required. When including a summary sheet, it should be included in the first Appendix attachment.
Applications that do not follow the Appendix requirements will not be reviewed.
Content:
You may include the following items in the Appendix (note, however, that some FOAs do not permit publications):
- Publications are not allowed as Appendix materials except in the circumstances noted below. When submitting an article, submit the entire article as a PDF attachment. Applicants may submit up to 3 of the following types of publications:
- Manuscripts and/or abstracts accepted for publication but not yet published.
- Published manuscripts and/or abstracts for which a free, online, publicly available journal link is not available.
- Patents directly relevant to the project: The entire document should be submitted as a PDF attachment.
- Surveys, questionnaires, and other data collection instruments; clinical protocols, and informed consent documents may be submitted in the Appendix.
Do not include the following items in the Appendix:
- Unpublished theses or abstracts/manuscripts submitted (but not yet accepted) for publication.
- Digital photographs or color images of gels, micrographs, etc. (These images must be included in the Research Strategy PDF). However, images embedded in publications are allowed.
- Publications that are publicly accessible. For such publications, the URL or PMC submission identification numbers, along with the full reference, should be included as appropriate in the Bibliography and References Cited section, the Progress Report Publication List section, and/or the Biographical Sketch section.
Additional Instructions for Multi-project:
Overall and Other Components: The "Appendix" attachment is optional.
Additional Instructions for SBIR/STTR:
Phase I SBIR/STTR Applications: Do not include appendices unless specifically solicited by NIH.
Format:
A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 Appendix attachments are needed, combine the remaining information into attachment #10. Note that this is the total number of Appendix items, not the total number of publications.
For materials that cannot be submitted electronically or materials that cannot be converted to PDF (e.g., medical devices, prototypes, DVDs, CDs), applicants should contact the Scientific Review Officer following notification of assignment of the application to a study section. Applicants are encouraged to be as concise as possible and submit only information essential for the review of the application.
Do not use the Appendix to circumvent the page limits of the Research Strategy or any other section of the application for which a page limit applies. For additional information regarding Appendix material and page limits, please refer to the NIH Guide Notice on Compliance with NIH Application Format and Content Instructions.
Use filenames for attachments that are descriptive of the content.
A summary sheet listing all of the items included in the Appendix is encouraged but not required. When including a summary sheet, it should be included in the first Appendix attachment.
Content:
The only allowable Appendix materials are:
For applications proposing clinical trials (unless the FOA provides other instructions for these materials):
- Clinical trial protocols
- Investigator's brochure from Investigational New Drug (IND), as appropriate.
For all applications:
- Blank informed consent/assent forms
- Blank surveys, questionnaires, data collection instruments
- FOA-specified items
- If Appendix materials are required in the FOA, review criteria for that FOA will address those materials, and applications submitted without those Appendix materials will be considered incomplete and will not be reviewed.
Note: Applications that do not follow the Appendix requirements will not be reviewed. Applications submitted for due dates on or after January 25, 2017 will be withdrawn and not reviewed if they are submitted with Appendix materials that are not specifically listed in this section.
For more Information:
- Information that expands upon or complements information provided in any section of the application - even if it is not required for the review - is not allowed in the Appendix unless it is listed in the allowed Appendix materials above. For more information, please see the NIH Guide Notice on Compliance with NIH Application Format and Content Instructions.
- Unless the FOA requires that certain information be included in the Appendix, failure of reviewers to address Appendix materials in their reviews is not an acceptable basis for an appeal of initial peer review. For more information, please see the NIH Guide Notice on Appeals of NIH Initial Peer Review.
Additional Instructions for Multi-project:
Overall and Other Components: The Appendix" attachment is optional.
Additional Instructions for SBIR/STTR:
Phase I SBIR/STTR Applications: Do not include appendices unless specifically solicited by NIH.
