Revised: December 7, 2018
G.410 - PHS 398 Career Development Award Supplemental Form
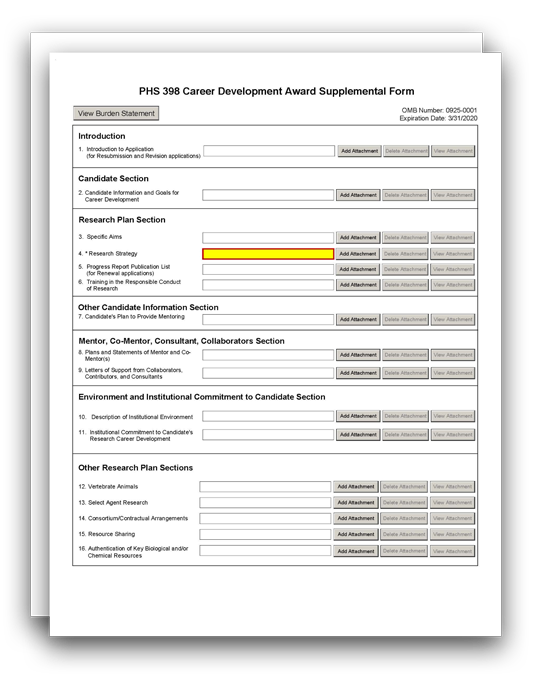
The PHS 398 Career Development Award Supplemental Form is used only for career development applications and multi-project applications with an "Indiv. Career Dev" Component.
This form includes fields to upload several attachments including the Specific Aims, Research Strategy, and Candidate Background and Goals.
See NIH's Reference Letters page for information including instructions for referees and how to submit letters.
The attachments in this form, together with the rest of your application, should include sufficient information needed for evaluation of the project and the candidate, independent of any other documents (e.g., previous application). Be specific and informative, and avoid redundancies.
- 3. Specific Aims
- 4. Research Strategy
- 5. Progress Report Publication List (for Renewal applications)
- 6. Training in the Responsible Conduct of Research
Mentor, Co-Mentor, Consultant, Collaborators Section
- 8. Plans and Statements of Mentor and Co-Mentor(s)
- 9. Letters of Support from Collaborators, Contributors, and Consultants
- 10. Description of Institutional Environment
- 11. Institutional Commitment to Candidate's Research Career Development
- 12. Vertebrate Animals
- 13. Select Agent Research
- 14. Consortium/Contractual Arrangements
- 15. Resource Sharing
- 16. Authentication of Key Biological and/or Chemical Resources
Citizenship
Who should use the PHS 398 Career Development Award Supplemental Form:
Use the PHS 398 Career Development Award Supplemental Form only if you are submitting a career development application or a multi-project application that has an "Indiv. Career Dev" Component.
Some sections of the PHS 398 Career Development Award Supplemental Form are required for all career development award applications, while others are to be used only when required by the FOA.
Read all the instructions in the FOA before completing this section to ensure your application meets all IC-specific criteria.
Applicants must follow all policies and requirements related to formatting, page limits, and proprietary information. See the following pages for more information:
- Format Attachments
- Page Limits
- NIH Grants Policy Statement, Section 2.3.11.2: Confidentiality of Information
- NIH Grants Policy Statement, Section 2.3.11.2.2: The Freedom of Information Act
Introduction
1. Introduction to Application (for Resubmission and Revision applications)
Who must complete the "Introduction to Application" attachment:
An "Introduction to Application" attachment is required only if the type of application is resubmission or revision. An introduction is not allowed for new or renewal applications.
Descriptions of different types of applications are listed here: NIH Types of Applications.
Format:
Follow the page limits for the Introduction in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Resubmission applications: See specific instructions on the content of the Introduction on the NIH's Resubmission Applications page.
Competing Revisions: See specific instructions on the content of the Introduction on the NIH's Competing Revisions page.
Additional Instructions for Multi-project:
Other Components: The "Introduction" attachment is optional for resubmissions and revisions applications. Although the "Introduction" attachment is optional, you may get a system warning if there is no attachment.
Candidate Section
2. Candidate Information and Goals for Career Development
Who must complete the "Candidate Information and Goals for Career Development" attachment:
The "Candidate Information and Goals for Career Development" attachment is required.
Format:
Follow the page limits for Candidate Information and Goals for Career Development in the NIH Table of Page Limits, unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Organize your attachment into three sections, following the headings and specified order below, and discuss each of the points listed below. Start each section with the appropriate section heading - Candidate's Background, Career Goals and Objectives, and Candidate's Plan for Career Development/Training Activities During Award Period. Also include any additional information requested in the FOA.
Candidate's Background:
- Describe your past scientific history, indicating how the award fits into past and future research career development.
- If there are consistent themes or issues that have guided previous work, these should be made clear. Alternatively, if your work has changed direction, indicate the reasons for the change.
Career Goals and Objectives:
- Describe your short-term and long-term career development goals.
- Justify the need for the award by describing how the career development award will enable you to develop and/or expand your research career.
- If applicable (e.g., K24), describe how this award will help you to serve as a mentor to early career investigators.
Candidate's Plan for Career Development/Training Activities During Award Period:
- Describe the new or enhanced research skills and knowledge you will acquire as a result of the proposed award.
- For non-mentored career development awards, describe any planned release from teaching, administrative, and/or clinical duties that will help you focus on your research activities, and if applicable, your mentoring activities.
- For mentored career development awards, describe any structured activities that are part of the developmental plan, such as coursework or workshops that will help you learn new techniques or develop needed professional skills.
- Briefly discuss each of the activities, other than research, in which you expect to participate.
- For each activity, other than research, explain how it relates to the proposed research and to the career development plan. Indicate the percentage of time to be dedicated to each activity by year, expressed in person months. For more information about calculating person months, see NIH's Frequently Asked Questions on Person Months.
- You are encouraged to include a timeline, including plans to apply for subsequent grant support.
Research Plan Section
A Research Plan is required for all types of individual career development awards.
The information in these introductory paragraphs to the Research Plan Section applies to all four Research Plan attachments: Specific Aims, Research Strategy, Progress Report Publication List, and Training in the Responsible Conduct of Research.
The Research Plan is a major part of the overall career development goal. It is important to relate the proposed research to the candidate's scientific career goals. Describe how the research, coupled with other developmental activities, will provide the experience, knowledge, and skills necessary to achieve the objectives of the career development plan. Also describe how the research and other developmental activities will enable the candidate to launch and conduct an independent research career or enhance an established research career.
For most types of research, the Research Plan Section should include:
- a specific hypothesis,
- a list of the specific aims and objectives that will be used to examine the hypothesis,
- a description of the methods/approaches/techniques to be used in each aim,
- a discussion of possible problems and how they will be managed, and
- alternative approaches that might be tried if the initial approaches do not work.
A Career Development Award (CDA) Research Plan is expected to be tailored to the experience level of the candidate and to allow him/her to develop the necessary skills needed for further career advancement. Reviewers will evaluate the plan accordingly. The plan should be achievable within the requested time period. Pilot or preliminary studies and routine data gathering are generally not appropriate as the sole part(s) of a CDA Research Plan.
Although candidates for mentored career development awards are expected to write the Research Plan, the mentor should review a draft of the plan and discuss it in detail with the candidate. Review by other knowledgeable colleagues is also helpful. Although it is understood that CDA applications do not require the extensive detail usually incorporated into regular research grant applications, a fundamentally sound Research Plan that includes a reasonably detailed Research Strategy section should be provided.
3. Specific Aims
Who must complete the "Specific Aims" attachment:
The "Specific Aims" attachment is required unless otherwise specified in the FOA.
Format:
Follow the page limits for the Specific Aims in the NIH Table of Page Limits, unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
State concisely the goals of the proposed research and summarize the expected outcome(s), including the impact that the results of the proposed research will have on the research field(s) involved.
List succinctly the specific objectives of the research proposed (e.g., to test a stated hypothesis, create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice, address a critical barrier to progress in the field, or develop new technology).
4. Research Strategy
Who must complete the "Research Strategy" attachment:
The "Research Strategy" attachment is required.
Format:
Follow the page limits for the Research Strategy in the NIH Table of Page Limits, unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
![]() Updated rigor instructions and review language take effect for all applications submitted for due dates on or after January 25, 2019. Use the correct set of instructions according to your application due date.The Research Strategy instructions will be changing, effective January 25, 2019. Please note that there are two sets of "Content" instructions below, based on the application due dates.
Updated rigor instructions and review language take effect for all applications submitted for due dates on or after January 25, 2019. Use the correct set of instructions according to your application due date.The Research Strategy instructions will be changing, effective January 25, 2019. Please note that there are two sets of "Content" instructions below, based on the application due dates.
For applications submitted for due dates on or before January 24, 2019:
Content:
Organize the Research Strategy in the specified order and use the instructions provided below. Start each section with the appropriate heading - Significance, Innovation, Approach.
Cite published experimental details in the Research Strategy section and provide the full reference in G.220 - R&R Other Project Information Form, Bibliography and References Cited.
In general, less detail will be expected in descriptions of research planned for the future years of the proposed CDA compared to the initial years' descriptions. However, sufficient detail should be provided to enable peer reviewers to determine that the plans for those years, including the approach to be used, are worthwhile and are likely to enable the candidate to achieve the objectives of the Research Plan.
Note for mentored CDA applications: Explain the relationship between the candidate's research on the CDA and the mentor's ongoing research program.
Note for non-mentored CDA applications: In general, non-mentored CDA applicants are expected to have independent, peer-reviewed research support. Applications should include a brief description of currently funded research, along with a more extensive description of any new research to be supported by the CDA.
![]() The new PHS Human Subjects and Clinical Trials Information form must be used for all applications submitted for due dates on or after January 25, 2018. That form will capture detailed study information for consolidated human subjects, inclusion enrollment report, and clinical trial information.Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
The new PHS Human Subjects and Clinical Trials Information form must be used for all applications submitted for due dates on or after January 25, 2018. That form will capture detailed study information for consolidated human subjects, inclusion enrollment report, and clinical trial information.Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
- Although some overall information may be duplicative between the Research Strategy and PHS Human Subjects and Clinical Trials Information form, it is usually inappropriate to copy/paste large blocks of text. Use the Research Strategy attachment to discuss the overall strategy, methodology, and analyses of your proposed research. Use the PHS Human Subjects and Clinical Trials Information form to provide detailed information for human subjects studies and clinical trials.
- The PHS Human Subjects and Clinical Trials Information form will capture detailed study information, including eligibility criteria; inclusion of women, minorities, and children; protection and monitoring plans; and statistical design and power.
- You are encouraged to refer to information in the PHS Human Subjects and Clinical Trials Information form as appropriate in your discussion of the Research Strategy (e.g., see Question 2.4 Inclusion of Women, Minorities, and Children).
Note for Applicants with Multiple Specific Aims: You may address the Significance, Innovation, and Approach either for each Specific Aim individually or for all of the Specific Aims collectively.
1. Significance
- Explain the importance of the problem or critical barrier to progress that the proposed project addresses.
- Describe the scientific premise for the proposed project, including consideration of the strengths and weaknesses of published research or preliminary data crucial to the support of your application.
- Explain how the proposed project will improve scientific knowledge, technical capability, and/or clinical practice in one or more broad fields.
- Describe how the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field will be changed if the proposed aims are achieved.
2. Innovation
- Explain how the application challenges current research or clinical practice paradigms.
- Describe any novel theoretical concepts, approaches or methodologies, instrumentation or interventions to be developed or used, and any advantage over existing methodologies, instrumentation, or interventions.
3. Approach
- Describe the overall strategy, methodology, and analyses to be used to accomplish the specific aims of the project. Describe the experimental design and methods proposed and how they will achieve robust and unbiased results. Unless addressed separately in the Resource Sharing Plan section, include how the data will be collected, analyzed, and interpreted, as well as any resource sharing plans as appropriate. Resources and tools for rigorous experimental design can be found at the Enhancing Reproducibility through Rigor and Transparency website.
- For trials that randomize groups or deliver interventions to groups, describe how your methods for analysis and sample size are appropriate for your plans for participant assignment and intervention delivery. These methods can include a group- or cluster-randomized trial or an individually randomized group-treatment trial. Additional information is available at the Research Methods Resources webpage.
- Discuss potential problems, alternative strategies, and benchmarks for success anticipated to achieve the aims.
- If the project is in the early stages of development, describe any strategy to establish feasibility, and address the management of any high risk aspects of the proposed work.
- Explain how relevant biological variables, such as sex, are factored into research designs and analyses for studies in vertebrate animals and humans. For example, strong justification from the scientific literature, preliminary data, or other relevant considerations, must be provided for applications proposing to study only one sex. Refer to NIH Guide Notice on Sex as a Biological Variable in NIH-funded Research for additional information.
- Point out any procedures, situations, or materials that may be hazardous to personnel and precautions to be exercised. A full discussion on the use of select agents should appear in the Select Agent Research section below.
- If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line from the NIH hESC Registry cannot be chosen, provide a strong justification for why an appropriate cell line cannot be chosen from the registry at this time.
- If you are proposing to gain clinical trial research experience (i.e., you will not be leading an independent clinical trial), briefly describe your role on the clinical trial.
As applicable, also include the following information as part of the Research Strategy, keeping within the three sections (Significance, Innovation, and Approach) listed above.
Preliminary Studies (for New Applications):
For new applications, include information on preliminary studies. Discuss the PD/PI's preliminary studies, data, and or experience pertinent to this application.
Progress Report (for Renewal and Revision Applications):
Most career development applicants will not complete this attachment. However, if you are required to do so, note that the Progress Report falls within the Research Strategy and is therefore included in the page limits for the Research Strategy.
For renewal/revision applications, provide a Progress Report. Provide the beginning and ending dates for the period covered since the last competitive review. In the Progress Report, you should:
- Summarize the specific aims of the previous project period and the importance of the findings, and emphasize the progress made toward their achievement.
- Explain any significant changes to the specific aims and any new directions, including changes resulting from significant budget reductions.
- Discuss previous participant enrollment (e.g., recruitment, retention, inclusion of women, minorities, children, etc.) for any studies meeting the NIH definition for clinical research. Use the Progress Report section to discuss, but not duplicate information collected elsewhere in the application.
Do not include a list of publications, patents, or other printed materials in the Progress Report. That information should be included in the "Progress Report Publication List" attachment.
![]() Updated rigor instructions and review language take effect for all applications submitted for due dates on or after January 25, 2019. Use the correct set of instructions according to your application due date.For applications submitted for due dates on or after January 25, 2019:
Updated rigor instructions and review language take effect for all applications submitted for due dates on or after January 25, 2019. Use the correct set of instructions according to your application due date.For applications submitted for due dates on or after January 25, 2019:
Content:
Organize the Research Strategy in the specified order and use the instructions provided below. Start each section with the appropriate heading - Significance, Innovation, Approach.
Cite published experimental details in the Research Strategy section and provide the full reference in G.220 - R&R Other Project Information Form, Bibliography and References Cited.
In general, less detail will be expected in descriptions of research planned for the future years of the proposed CDA compared to the initial years' descriptions. However, sufficient detail should be provided to enable peer reviewers to determine that the plans for those years, including the approach to be used, are worthwhile and are likely to enable the candidate to achieve the objectives of the Research Plan.
Note for mentored CDA applications: Explain the relationship between the candidate's research on the CDA and the mentor's ongoing research program.
Note for non-mentored CDA applications: In general, non-mentored CDA applicants are expected to have independent, peer-reviewed research support. Applications should include a brief description of currently funded research, along with a more extensive description of any new research to be supported by the CDA.
![]() The new PHS Human Subjects and Clinical Trials Information form must be used for all applications submitted for due dates on or after January 25, 2018. That form will capture detailed study information for consolidated human subjects, inclusion enrollment report, and clinical trial information.Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
The new PHS Human Subjects and Clinical Trials Information form must be used for all applications submitted for due dates on or after January 25, 2018. That form will capture detailed study information for consolidated human subjects, inclusion enrollment report, and clinical trial information.Note for Applications Proposing the Involvement of Human Subjects and/or Clinical Trials:
- Although some overall information may be duplicative between the Research Strategy and PHS Human Subjects and Clinical Trials Information form, it is usually inappropriate to copy/paste large blocks of text. Use the Research Strategy attachment to discuss the overall strategy, methodology, and analyses of your proposed research. Use the PHS Human Subjects and Clinical Trials Information form to provide detailed information for human subjects studies and clinical trials.
- The PHS Human Subjects and Clinical Trials Information form will capture detailed study information, including eligibility criteria; inclusion of women, minorities, and children; protection and monitoring plans; and statistical design and power.
- You are encouraged to refer to information in the PHS Human Subjects and Clinical Trials Information form as appropriate in your discussion of the Research Strategy (e.g., see Question 2.4 Inclusion of Women, Minorities, and Children).
Note for Applicants with Multiple Specific Aims: You may address the Significance, Innovation, and Approach either for each Specific Aim individually or for all of the Specific Aims collectively.
1. Significance
- Explain the importance of the problem or critical barrier to progress that the proposed project addresses.
- Describe the strengths and weaknesses in the rigor of the prior research (both published and unpublished) that serves as the key support for the proposed project.
- Explain how the proposed project will improve scientific knowledge, technical capability, and/or clinical practice in one or more broad fields.
- Describe how the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field will be changed if the proposed aims are achieved.
2. Innovation
- Explain how the application challenges current research or clinical practice paradigms.
- Describe any novel theoretical concepts, approaches or methodologies, instrumentation or interventions to be developed or used, and any advantage over existing methodologies, instrumentation, or interventions.
3. Approach
- Describe the overall strategy, methodology, and analyses to be used to accomplish the specific aims of the project. Describe plans to address weaknesses in the rigor of the prior research that serves as the key support for the proposed project. Describe the experimental design and methods proposed and how they will achieve robust and unbiased results. Unless addressed separately in the Resource Sharing Plan section, include how the data will be collected, analyzed, and interpreted, as well as any resource sharing plans as appropriate. Resources and tools for rigorous experimental design can be found at the Enhancing Reproducibility through Rigor and Transparency website.
- For trials that randomize groups or deliver interventions to groups, describe how your methods for analysis and sample size are appropriate for your plans for participant assignment and intervention delivery. These methods can include a group- or cluster-randomized trial or an individually randomized group-treatment trial. Additional information is available at the Research Methods Resources webpage.
- Discuss potential problems, alternative strategies, and benchmarks for success anticipated to achieve the aims.
- If the project is in the early stages of development, describe any strategy to establish feasibility, and address the management of any high risk aspects of the proposed work.
- Explain how relevant biological variables, such as sex, are factored into research designs and analyses for studies in vertebrate animals and humans. For example, strong justification from the scientific literature, preliminary data, or other relevant considerations, must be provided for applications proposing to study only one sex. Refer to NIH Guide Notice on Sex as a Biological Variable in NIH-funded Research for additional information.
- Point out any procedures, situations, or materials that may be hazardous to personnel and precautions to be exercised. A full discussion on the use of select agents should appear in the Select Agent Research section below.
- If research on Human Embryonic Stem Cells (hESCs) is proposed but an approved cell line from the NIH hESC Registry cannot be chosen, provide a strong justification for why an appropriate cell line cannot be chosen from the registry at this time.
- If you are proposing to gain clinical trial research experience (i.e., you will not be leading an independent clinical trial), briefly describe your role on the clinical trial.
As applicable, also include the following information as part of the Research Strategy, keeping within the three sections (Significance, Innovation, and Approach) listed above.
Preliminary Studies (for New Applications):
For new applications, include information on preliminary studies. Discuss the PD/PI's preliminary studies, data, and or experience pertinent to this application.
Progress Report (for Renewal and Revision Applications):
Most career development applicants will not complete this attachment. However, if you are required to do so, note that the Progress Report falls within the Research Strategy and is therefore included in the page limits for the Research Strategy.
For renewal/revision applications, provide a Progress Report. Provide the beginning and ending dates for the period covered since the last competitive review. In the Progress Report, you should:
- Summarize the specific aims of the previous project period and the importance of the findings, and emphasize the progress made toward their achievement.
- Explain any significant changes to the specific aims and any new directions, including changes resulting from significant budget reductions.
- Discuss previous participant enrollment (e.g., recruitment, retention, inclusion of women, minorities, children, etc.) for any studies meeting the NIH definition for clinical research. Use the Progress Report section to discuss, but not duplicate information collected elsewhere in the application.
Do not include a list of publications, patents, or other printed materials in the Progress Report. That information should be included in the "Progress Report Publication List" attachment.
5. Progress Report Publication List (for Renewal applications)
Who must complete the "Progress Report Publication List" attachment:
A "Progress Report Publication List" attachment is required only if the type of application is renewal. Most career development applicants will not complete this attachment.
Descriptions of different types of applications are listed here: NIH's Types of Applications.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
List the titles and complete references to all appropriate publications, manuscripts accepted for publication, patents, and other printed materials that have resulted from the project since it was last reviewed competitively.
You are allowed to cite interim research products. Note: interim research products have specific citation requirements. See related Frequently Asked Questions on citing interim research products and claiming them as products of your NIH award.
Provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for the following:
- Articles that fall under the Public Access Policy,
- Articles that were authored or co-authored by the applicant and arose from NIH support,
- Articles that were authored or co-authored by the applicant and arose from AHRQ funding provided after February 19, 2016 (see the Guide Notice on Policy for Public Access to AHRQ-Funded Scientific Publications).
If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate "PMC Journal - In Process." NIH maintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PubMed ID (PMID) numbers along with the full reference.
Additional Instructions for Multi-project:
Overall and Other Components: If you include a "Progress Report Publication List" attachment, you can include it in either the Overall Component or within each Other Component, but do not attach the same information in multiple locations.
6. Training in the Responsible Conduct of Research
Who must complete the "Training in the Responsible Conduct of Research" attachment:
The "Training in the Responsible Conduct of Research" attachment is required.
Format:
Follow the page limits for the Training in the Responsible Conduct of Research in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Mentored CDA applications should describe a plan to acquire instruction in the responsible conduct of research (RCR).
Non-mentored (independent) CDA applications should describe a plan to obtain or provide instruction in RCR, depending on your level of experience with RCR.
Attach a description of plans for obtaining or providing instruction in RCR. This section should document prior instruction or participation in RCR training during the applicant's current career stage (including the date instruction was last completed). This section should also propose plans to either receive instruction or provide instruction (e.g., to participate as a course lecturer) to meet the frequency requirement of RCR training (see the "For more information section" below).
The plan must address the five required instructional components outlined in the NIH Policy on Instruction in the Responsible Conduct of Research (RCR), as more fully described in the NIH Grants Policy Statement, Section 12.4.1.4: Training in the Responsible Conduct of Research.
- Format: Describe the required format of instruction, i.e., face-to-face lectures, coursework, and/or real-time discussion groups (a plan with only on-line instruction is not acceptable).
- Subject Matter: Describe the breadth of subject matter (e.g., conflict of interest, authorship, data management, human subjects and animal use, laboratory safety, research misconduct, research ethics).
- Faculty Participation: Describe the role of the mentor(s) and other faculty involvement in the instruction.
- Duration of Instruction: Describe the number of contact hours of instruction, taking into consideration the duration of the program.
- Frequency of Instruction: Instruction must occur during each career stage and at least once every four years. Document any prior instruction during the applicant's current career stage, including the inclusive dates instruction was last completed.
The plan may include career stage-appropriate individualized instruction or independent scholarly activities. Instruction and activities should enhance the applicant's understanding of ethical issues related to their specific research activities and the societal impact of that research. The role of the mentor in RCR instruction must be described.
Renewal Applications: Describe the RCR instruction activities undertaken during the previous project period as well as future plans for RCR instruction.
For more information:
See the NIH Grants Policy Statement, Section 12.4.1.4: Training in the Responsible Conduct of Research.
Other Candidate Information Section
7. Candidate's Plan to Provide Mentoring
Who must complete the "Candidate's Plan to Provide Mentoring" attachment:
Include the "Candidate's Plan to Provide Mentoring" attachment only when required by the FOA, (e.g., K05 and K24).
Format:
Follow the page limits for the Candidate's Plan to Provide Mentoring in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The plan should provide information about both the candidate's commitment to serve as a mentor to other investigators and the candidate's previous mentoring activities. State the candidate's proposed percent effort commitment to the mentoring plan, expressed in person months. For more information about calculating person months, see NIH's Frequently Asked Questions on Person Months.
Describe proposed mentoring activities: Describe the setting for mentoring and provide information about the available pool of mentees with appropriate backgrounds and similar interests in science as the candidate. Include information sufficient for reviewers to evaluate the quality of the proposed mentoring experience, including the professional levels of mentees and the frequency and kinds of mentoring interactions between the candidate and mentees. Describe the productivity of the mentoring relationship for the scientific development of the new scientists as judged by their publications and current research activities.
Describe past mentoring activities: Include sufficient information on the candidate's past mentees so that reviewers can evaluate the quality of prior mentoring experiences. Include information such as the professional levels of mentees, and the frequency and kinds of mentoring interactions between the candidate and mentees.
Senior level (K05) candidates: Describe any financial and material support from your own funded research and research resources that will be available to your mentees.
Mentor, Co-Mentor, Consultant, Collaborators Section
8. Plans and Statements of Mentor and Co-Mentor(s)
Who must complete the "Plans and Statements of Mentor and Co-Mentor(s)" attachment:
Any candidate applying for a mentored CDA (see Summary of Career Development Award Mechanisms table) must include a "Plans and Statement of Mentor and Co-Mentor(s)" attachment.
All mentored career development applications should identify any and all co-mentors involved with the proposed research and career development program. The mentor and each co-mentor must provide a statement as described below.
Format:
Follow the page limits for the Plans and Statements of Mentor and Co-mentor(s) in the NIH Table of Page Limits unless otherwise specified in the FOA.
The plans and statements must be appended together and uploaded as a single PDF file. See NIH's Format Attachments page.
Content:
The mentor and co-mentor(s) (if applicable) must each document their role and willingness to participate in the project, and explain how they will contribute to the development of the candidate's research career. Each statement should include all of the following:
- The plan for the candidate's training and research career development. Include information not only about research, but also about other developmental activities, such as seminars, scientific meetings, training in RCR, and presentations. Discuss expectations for publications over the entire period of the proposed project. Define what aspects of the proposed research project the candidate will be allowed to continue to pursue as part of his/her independent research program.
- The source of anticipated support for the candidate's research project for each year of the award period.
- The nature and extent of supervision and mentoring of the candidate, and commitment to the candidate's development that will occur during the award period.
- The candidate's anticipated teaching load for the award period (number and types of courses or seminars), clinical responsibilities, committee and administrative assignments, and the portion of time available for research.
- A plan for transitioning the candidate from the mentored stage of his/her career to the independent investigator stage by the end of the project period of the award. Describe the mentor's (or co-mentor's) previous experience as a mentor, including type of mentoring (e.g., graduate students, career development awardees, postdoctoral fellows), number of persons mentored, and career outcomes.
Note for co-mentor statements: Co-mentors must also address the nature of their role in the career development plan and how the responsibility for the candidate's development is shared with the mentor. Describe respective areas of expertise and how they will be combined to enhance the candidate's development. Also describe the nature of any resources that will be committed to this CDA.
Note: If the applicant is proposing to gain experience in a clinical trial as part of his or her research career development, then the mentor or a member of the mentoring team should include information in the statement to document leadership of the clinical trial (in addition to the information above). Include the following:
- Source of funding;
- ClinicalTrials.gov Identifier (e.g., NCT87654321), if applicable;
- A description of how your expertise is appropriate to guide the applicant in any proposed clinical trials research experience; and
- A statement/attestation that the mentor will be responsible for the clinical trial.
- The mentor must have primary responsibility for leading and overseeing the trial and must describe how she/he will provide this oversight (be careful not to overstate the candidate's responsibilities).
- Include details on the specific roles/responsibilities of the applicant and mentor, keeping in mind that the terms of a CDA award do not always permit the candidate to lead a clinical trial.
Do not place these statements from the mentor(s) and co-mentor(s) in the Appendix.
9. Letters of Support from Collaborators, Contributors, and Consultants
Note that letters of support are not the same as letters of reference (also known as reference letters), which are required for some K applications. For more information about letters of reference, see the NIH's Reference Letters page.
From whom are letters of support required? From whom are letters not required?
Letters of support from collaborators, contributors, and consultants will be required for any such person who will contribute to the scientific development or execution of CDA application's proposed project. Follow the requirements for letters of support as listed in the FOA.
Letters are not required for personnel (such as research assistants) not contributing in a substantive, measurable way to the scientific development or execution of the project.
Format:
Follow the page limits for the Letters of Support from Collaborators, Contributors, and Consultants in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach all appropriate letters of support. The letters must be appended together and uploaded as a single PDF file. See NIH's Format Attachments page.
Content:
Letters from consultants should include rates/charges for consulting services.
Mentored CDA applications should identify collaborators, contributors, and consultants involved with the proposed research and career development program, and not already included in the "Plans and Statements of Mentor(s) and Co-Mentor(s)" section. Letters should briefly describe their anticipated contributions and document their role and willingness to participate in the project. The letters should also briefly describe research materials, data, guidance, or advice each person will provide.
Non-mentored CDA applications should include letters from collaborators, consultants, and contributors. Letters should list proposed roles and document their willingness to participate in the project. The letters should also briefly describe research materials, data, guidance, or advice each person will provide.
Environment and Institutional Commitment to Candidate Section
10. Description of Institutional Environment
Who must complete the "Description of Institutional Environment" attachment:
The "Description of Institutional Environment" attachment is required.
Format:
Follow the page limits for the Description of Institutional Environment in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Mentored CDA applicants: Describe the institution's research and career development opportunities related to your area(s) of interest, including the names of key faculty members and other investigators relevant to your proposed developmental plan and capable of productive collaboration with the candidate. Indicate how the necessary facilities and other resources will be made available for both career enhancement and the research proposed in this application - refer to the resources description in G.220 - R&R Other Project Information Form, Facilities and Other Resources in your "Description of Institutional Environment" attachment. Describe opportunities for intellectual interactions with other investigators, including courses offered, journal clubs, seminars, and presentations.
Non-mentored CDA applicants: Describe the institution's research and career development opportunities related to your area(s) of interest, including the names of other faculty members who are willing to collaborate with you. Indicate how the necessary facilities and other resources will be made available for both career enhancement and the research proposed in this application - refer to the resources description in G.220 - R&R Other Project Information Form, Facilities and Other Resources in your "Description of Institutional Environment" attachment. Describe opportunities for intellectual interactions with other investigators, including journal clubs, seminars, and presentations.
11. Institutional Commitment to Candidate's Research Career Development
Who must complete the "Institutional Commitment to Candidate's Research Career Development" attachment:
The "Institutional Commitment to Candidate's Research Career Development" attachment is required.
Format:
Follow the page limits for the Institutional Commitment to Candidate's Research Career Development in the NIH Table of Page Limits unless otherwise specified in the FOA.
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
The sponsoring institution must provide a document on institutional letterhead that describes its commitment to the candidate and the candidate's career development, independent of the receipt of the CDA. It is also essential to document the institution's commitment to the retention, development, and advancement of the candidate during the period of the award.
The "Institutional Commitment to Candidate's Research Career Development" attachment should generally document the institution's agreement to provide adequate time, support, equipment, facilities, and resources to the candidate for research and career development activities. See the list below for specific items to include in the document.
In the document describing its institutional commitment, the applicant organization must:
- Agree to release the candidate from other duties and activities so that the candidate can devote the required percentage of time for development of a research career, as specified by the FOA. For most K awards, commitment of at least 75 percent or nine person months of time is required.
- NIH and other PHS agencies use the concept of "person months" as a metric for determining percent of effort. For more information about calculating person months, see NIH's Frequently Asked Questions on Person Months.
- Describe actions that will be taken to ensure that the candidate can devote the required time to research career development (e.g., reduction of the candidate's teaching load, committee and administrative assignments, and clinical or other professional activities for the current academic year). If the candidate's clinical or teaching responsibilities will be reduced, describe how this will be accommodated (e.g., hiring additional staff, reassigning staff, etc.).
- Describe the candidate's academic appointment, bearing in mind that the appointment must be full-time, and that the appointment (including all rights and privileges pertaining to full faculty status if in an academic setting) and the continuation of salary should not be contingent upon the receipt of this award.
- Describe the proportion of time currently available for the candidate's research and what the candidate's institutional responsibilities will be if an award is made.
- Describe how the institution will provide the candidate with appropriate office and laboratory space, equipment, and other resources (including access to clinical and/or other research populations) to carry out the proposed Research Plan.
- Describe how the institution will be supportive of any proposed mentor(s), other staff, and/or collaborations with other faculty consistent with the career development plan.
Signatures:
The institutional commitment must be dated and signed by the person who is authorized to commit the institution to the agreements and assurances listed above. In most cases, this will be the dean or the chairman of the department. The signature must appear over the signer's name and title at the end of the statement. If the candidate will be working outside of the applicant institution (i.e., sponsoring institution), signatures from both the applicant/sponsoring institution and host institutions are required.
The sponsoring institution, through the submission of the application and in the institutional commitment section, certifies that all items outlined above will be provided and that the institution will abide by the applicable assurances and PHS policies.
Note: For applicable assurances, see the NIH Grants Policy Statement, Section 4: Public Policy Requirements, Objectives and Other Appropriation Mandates.
Other Research Plan Sections
12. Vertebrate Animals
Who must complete the "Vertebrate Animals" attachment:
Include the "Vertebrate Animals" attachment if you answered "Yes" to the question "Are Vertebrate Animals Used?" on the G.220 - R&R Other Project Information Form.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Do not use the Vertebrate Animals attachment to circumvent the page limits of the Research Strategy.
Content:
If live vertebrate animals are involved in the project, address each of the following criteria:
- Description of Procedures: Provide a concise description of the proposed procedures to be used that involve live vertebrate animals in the work outlined in the "Research Strategy" attachment. The description must include sufficient detail to allow evaluation of the procedures. Identify the species, strains, ages, sex, and total numbers of animals by species, to be used in the proposed work. If dogs or cats are proposed, provide the source of the animals.
- Justifications: Provide justification that the species are appropriate for the proposed research. Explain why the research goals cannot be accomplished using an alternative model (e.g. computational, human, invertebrate, in vitro).
- Minimization of Pain and Distress: Describe the interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to minimize discomfort, distress, pain, and injury.
Each of the criteria must be addressed. Failure to adequately address the criteria may negatively affect the application's impact score. In addition to the 3 criteria above, you should also:
- Identify all project performance (or collaborating) sites and describe the proposed research activities with vertebrate animals that will be conducted at those sites.
- Explain when and how animals are expected to be used if plans for the use of animals have not been finalized.
See the following pages for more information:
- NIH's Office of Laboratory Animal Welfare website
- NIH's Vertebrate Animals Section Worksheet
- NIH Grants Policy Statement, Section 4.1.1: Animal Welfare Requirements (an applicable Animal Welfare Assurance will be required if the grantee institution does not have one)
13. Select Agent Research
Who must complete the "Select Agent Research" attachment:
Include the "Select Agent Research" attachment if your proposed activities involve the use of select agents at any time during the proposed project period, either at the applicant organization or at any performance site.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
For more information:
Select agents are hazardous biological agents and toxins that have been identified by HHS or the U.S. Department of Agriculture (USDA) as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. The Centers for Disease Control and Prevention (CDC) and the Animal APHIS Select Agent Programs jointly maintain a list of these agents. See the Federal Select Agent Program website.
Content:
Excluded select agents: If the activities proposed in your application involve only the use of a strain(s) of select agents which has been excluded from the list of select agents and toxins as per 42 CFR 73.3, the select agent requirements do not apply. Use this "Select Agent Research" section to identify the strain(s) of the select agent that will be used and note that it has been excluded from this list. The CDC maintains a list of exclusions which is available on the Select Agents and Toxins Exclusions website.
Applying for a select agent to be excluded: If the strain(s) is not currently excluded from the list of select agents and toxins but you have applied or intend to apply to HHS for an exclusion from the list, use this section to indicate the status of your request or your intent to apply for an exclusion and provide a brief justification for the exclusion.
All applicants proposing to use select agents: Address the following three points for each site at which select agent research will take place. Although no specific page limitation applies to this section, be succinct.
- Identify the select agent(s) to be used in the proposed research.
- Provide the registration status of all entities* where select agent(s) will be used.
- If the performance site(s) is a foreign institution, provide the name(s) of the country or countries where select agent research will be performed.
- *An "entity" is defined in 42 CFR 73.1 as "any government agency (Federal, State, or local), academic institution, corporation, company, partnership, society, association, firm, sole proprietorship, or other legal entity."
- Provide a description of all facilities where the select agent(s) will be used.
- Describe the procedures that will be used to monitor possession, use and transfer of select agent(s).
- Describe plans for appropriate biosafety, biocontainment, and security of the select agent(s).
- Describe the biocontainment resources available at all performance sites.
14. Consortium/Contractual Arrangements
Who must complete the "Consortium/Contractual Arrangements" attachment:
Include the "Consortium/Contractual Arrangements" attachment if you have consortium/contracts in your budget.
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Explain the programmatic, fiscal, and administrative arrangements to be made between the applicant organization and the consortium organization(s). If consortium/contractual activities represent a significant portion of the overall project, explain why the applicant organization, rather than the ultimate performer of the activities, should be the grantee.
Note: The signature of the authorized organization representative in G.200 - SF 424 (R&R), Authorized Representative signifies that the applicant and all proposed consortium participants understand and agree to the following statement:
The appropriate programmatic and administrative personnel of each organization involved in this grant application are aware of the agency's consortium agreement policy and are prepared to establish the necessary inter-organizational agreement(s) consistent with that policy.
For more information:
Refer to the NIH Grants Policy Statement, Section 15: Consortium Agreements for more information.
Additional Instructions for Multi-project:
Overall and Other Components: Unless otherwise specified in the FOA, you have the option to:
- include a single consolidated "Consortium/Contractual Arrangements" attachment in the Overall Component, or
- include component-specific "Consortium/Contractual Arrangements" attachment(s) within the components that include subawards, or
- include a "Consortium/Contractual Arrangements" attachment in the Overall Component and include component-specific attachments within the components that include subawards. Each filename must be unique.
15. Resource Sharing
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
Data Sharing Plan: Investigators seeking $500,000 or more in direct costs (exclusive of consortium F&A) in any budget period are expected to include a brief 1-paragraph description of how final research data will be shared, or explain why data-sharing is not possible (for example human subject concerns, the Small Business Innovation Development Act provisions, etc.). Specific FOAs may require that all applications include this information regardless of the dollar level. Applicants are encouraged to read the FOA carefully and discuss their data-sharing plan with their program contact at the time they negotiate an agreement with the Institute/Center (IC) staff to accept assignment of their application. For more information, see the NIH Data Sharing Policy or the NIH Grants Policy Statement, Section 8.2.3.1: Data Sharing Policy.
Sharing Model Organisms: Regardless of the amount requested, all applications where the development of model organisms is anticipated are expected to include a description of a specific plan for sharing and distributing unique model organisms or state why such sharing is restricted or not possible. For more information, see the NIH Grants Policy Statement, Section 8.2.3.2: Sharing Model Organisms.
Genomic Data Sharing (GDS): Applicants seeking funding for research that generates large-scale human or non-human genomic data are expected to provide a plan for sharing of these data. Examples of large-scale genomic data include genome-wide association studies (GWAS), single nucleotide polymorphisms (SNP) arrays, and genome sequence, transcriptomic, epigenomic, and gene expression data. Supplemental Information to the NIH GDS provides examples of genomic research projects that are subject to the Policy. For more information, see the NIH GDS Policy, the NIH Grants Policy Statement, Section 8.2.3.3: Genomic Data Sharing (GDS) Policy/ Policy for Genome-Wide Association Studies (GWAS), and the GDS website.
Note on GDS: For proposed studies generating human genomic data under the scope of the GDS Policy, an Institutional Certification may be submitted at the time of application submission, but it is not required at that time. The Institutional Certification, however, will be requested as Just-in-Time (JIT) information prior to award. The Institutional Certification, or in some cases, a Provisional Institutional Certification, must be submitted and accepted before the award can be issued.
For more information:
NIH considers the sharing of unique research resources developed through NIH-sponsored research an important means to enhance the value and further the advancement of the research. When resources have been developed with NIH funds, and the associated research findings published or provided to NIH, it is important that they be made readily available for research purposes to qualified individuals within the scientific community. See the NIH Grants Policy Statement, Section 8.2.3: Sharing Research Resources.
16. Authentication of Key Biological and/or Chemical Resources
Format:
Attach this information as a PDF file. See NIH's Format Attachments page.
Content:
If applicable to the proposed science, briefly describe methods to ensure the identity and validity of key biological and/or chemical resources used in the proposed studies. A maximum of one page is suggested.
More information:
Key biological and/or chemical resources are characterized as follows:
- Key biological and/or chemical resources may or may not be generated with NIH funds and: 1) may differ from laboratory to laboratory or over time; 2) may have qualities and/or qualifications that could influence the research data; and 3) are integral to the proposed research. These include, but are not limited to, cell lines, specialty chemicals, antibodies, and other biologics.
- Standard laboratory reagents that are not expected to vary do not need to be included in the plan. Examples are buffers and other common biologicals or chemicals.
- See NIH's page on Rigor and Reproducibility for more information.
Appendix
17. Appendix
Refer to the FOA to determine whether there are any special appendix instructions for your application. See the updated NIH Guide Notice on the Appendix Policy.
Additional Instructions for Multi-project:
Overall and Other Components: The "Appendix" attachment is optional.
Format:
A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 allowable appendix attachments are needed, combine the remaining information into attachment #10.
Use filenames for attachments that are descriptive of the content.
A summary sheet listing all of the items included in the Appendix is encouraged but not required. When including a summary sheet, it should be included in the first appendix attachment.
Content:
The only allowable appendix materials are:
- Blank data collection forms, blank survey forms, and blank questionnaire forms - or screenshots thereof
- Simple lists of interview questions
Note: In your blank forms and lists, do not include items such as: data, data compilations, lists of variables or acronyms, data analyses, publications, manuals, instructions, descriptions or drawings/figures/diagrams of data collection methods or machines/devices.
- Blank informed consent/assent forms
- Other items only if they are specified in the FOA as allowable appendix materials
No other items are allowed in the Appendix. Simply relocating disallowed materials to other parts of the application will result in a noncompliant application.
Some FOAs may have different instructions for the Appendix. Always follow the instructions in your FOA if they conflict with these instructions.
Note: Applications will be withdrawn and not reviewed if they do not follow the appendix requirements in these instructions or in your FOA.
Information that expands upon or complements information provided in any section of the application - even if it is not required for the review - is not allowed in the Appendix unless it is listed in the allowed appendix materials above or in your FOA. For example, do not include material transfer agreements (MTA) in the appendix unless otherwise specified in the FOA.
For more information:
- The NIH Guide Notice on Reminder: NIH Applications Must Be Complete and Compliant With NIH Policy and Application Instructions At Time of Submission.
- Failure of reviewers to address non-required appendix materials in their reviews is not an acceptable basis for an appeal of initial peer review. For more information, see the NIH Grants Policy Statement, Section 2.4.2: Appeals of Initial Scientific Review.
- Appendix Policy Frequently Asked Questions
Citizenship
Information on Citizenship Requirements for CDA Applicants:
The candidate must be a citizen or non-citizen national of the United States or its possessions and territories, or must have been lawfully admitted to the United States for permanent residence by the time of award EXCEPT if any of the following apply:
- candidate is applying to the K99/R00 award program;
- candidate is applying to the K43 award program; or
- the FOA specifies otherwise.
Note for permanent residents: Before an award is issued, a permanent resident will be required to submit a notarized statement that the candidate holds a current and valid Permanent Resident Card or some other valid verification from the U.S. Immigration and Naturalization Service of legal admission to the U.S. as a permanent resident.
Note for candidates whose citizenship status changes or is expected to change: For those career development award programs that require candidates to be U.S. citizens or permanent residents, an individual who has applied for permanent residence and expects to have obtained such status prior to the time award may submit an application recognizing that no award will be made until legal verification of permanent resident status is provided. If a candidate's citizenship status changes after submission of the application, the new status should be reported in the candidate's Personal Profile in the eRA Commons.
Note on K99/R00 applicants on temporary visas: It is the responsibility of the applicant organization to determine and document in the application that the candidate's visa will allow him or her to remain in the U.S. long enough to complete the phase of the award (e.g., K99 or R00) covered by the application. Information may be requested by the NIH or another PHS Agency prior to issuance of an award as a Just-in-Time submission.
Check the applicable boxes for the following questions:
18. U.S. Citizen or Non-Citizen National?
Check "Yes" if the candidate is either a U.S. Citizen or a Non-Citizen national; otherwise check "No."
Non-Citizen nationals are people who, although not citizens of the United States, owe permanent allegiance to the United States. They generally are people born in outlying possessions of the United States (e.g., American Samoa and Swains Island).
If no, select most appropriate Non-U.S. Citizen option:
Please select the most appropriate response from the options provided.
With a Permanent U.S. Resident Visa:
Check this box if the candidate has been lawfully admitted for permanent residence (i.e., is in the possession of a current and valid Permanent Resident Card or other legal verification of such status). A notarized statement will be required as part of the pre-award process.
With a Temporary U.S. Visa:
Check this box if the candidate currently holds a temporary U.S visa. This box is applicable only to specific programs that do not require U.S. citizenship or permanent residency (e.g., K99/R00).
Not Residing in the U.S.:
Check this box if the candidate is a citizen of a country other than the U.S. and plans to pursue career development outside of the U.S. This box is applicable only to specific programs (e.g., K43).
If you are a non-U.S. citizen with a temporary visa applying for an award that requires permanent residency status, and expect to be granted a permanent resident visa by the start date of the award, check here:
Check this box to indicate that permanent resident status is pending (i.e., if the candidate is not a U.S citizen but has applied for permanent residence and expects to hold a permanent resident visa by the earliest possible start date of the award). A notarized statement will be required as a part of the pre-award process. The statement must show that a licensed notary has seen the career development applicant's valid Permanent Resident Card (USCIS Form I-551) or other valid verification from the U.S. Immigration and Naturalization Service of legal admission to the U.S.
