Participating Organization(s) |
National Institutes of Health (NIH) |
National Heart, Lung, and Blood Institute (NHLBI) |
|
Funding Opportunity Title |
Core Clinical Centers for the Cardiothoracic Surgical Trials Network, CTSN (UM1) |
Activity Code |
UM1 Multi-Component Research Project Cooperative Agreements |
Announcement Type |
Reissue of RFA-HL-06-005 |
Related Notices |
|
Funding Opportunity Announcement (FOA) Number |
RFA-HL-13-017 |
Companion FOA |
RFA-HL-13-022, Cardiothoracic Surgical Trials Network (CTSN) Limited Competition for the Data Coordinating Center (U01) |
Catalog of Federal Domestic Assistance (CFDA) Number(s) |
93.837 |
FOA Purpose |
The purpose of this FOA is to request applications for participation as a Core Clinical Center in a continuation of the Cardiothoracic Surgical Trials Network (CTSN). Highly innovative approaches to patient recruitment and retention for challenging cardiothoracic (CT) surgical trials, as well as a strong track record of prior trial performance, are sought. A separate solicitation (RFA-HL-13-022) seeks applications for a network Data Coordinating Center. The goal of the CTSN is to evaluate CT surgical interventions and related management approaches for the treatment of cardiovascular disease and conditions in adult patients. The network provides support to maintain an infrastructure to develop, coordinate, and conduct multiple collaborative surgical trials designed to improve cardiovascular outcomes and optimize post-operative neurological function. |
Posted Date |
March 28, 2012 |
Letter of Intent Due Date |
May 13, 2012 |
Application Due Date(s) |
June 13, 2012 |
AIDS Application Due Date(s) |
Not Applicable |
Scientific Merit Review |
|
Advisory Council Review |
|
Earliest Start Date(s) |
April 1, 2013 |
Expiration Date |
June 14, 2012 |
Due Dates for E.O. 12372 |
Not Applicable |
Required Application Instructions
It is critical that applicants follow the instructions in the PHS398 Application Guide except where instructed to do otherwise (in this FOA or in a Notice from the NIH Guide for Grants and Contracts). Conformance to all requirements (both in the Application Guide and the FOA) is required and strictly enforced. While some links are provided, applicants must read and follow all application instructions in the Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the Application Guide, follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
Part 1. Overview Information
Part 2. Full Text of Announcement
Section I. Funding Opportunity Description
Section II. Award Information
Section III. Eligibility Information
Section IV. Application and Submission
Information
Section V. Application Review Information
Section VI. Award Administration Information
Section VII. Agency Contacts
Section VIII. Other Information
Nature of the Research Opportunity
In 2007, the National Heart, Lung and Blood Institute, in collaboration with the Canadian Institutes of Health Research, and the National Institute for Neurological Disorders and Stroke, launched the CardioThoracic Surgical trials Network (CTSN), to develop and conduct studies, mostly randomized controlled trials, to evaluate surgical interventions and related management approaches, for the treatment of cardiovascular diseases. The network was established to provide the rigorous evidence base upon which key clinical treatment strategies could ultimately be based.
However, the clinical research enterprise is generally challenging and frequent difficulties with patient recruitment and retention into trials are experienced. It is well known that surgical trials are especially challenging for a variety of reasons. The CTSN has not been immune to such difficulties and although current trials are anticipated to complete enrollment within the initial grant budget, there remains a strong need for better approaches to identify, recruit, randomize, and retain patients and logistically manage cardiothoracic surgical trials. Thus, the objective of this funding opportunity announcement is to solicit for experienced Core Clinical Centers (CCCs) that propose innovative strategies to successfully operate trials within the purview of the CTSN.
This solicitation requests applications for CCCs to support a 5-year renewal of the CTSN. With the start of the renewal period in 2013, it is anticipated that the network will have up to eight CCCs. Applications from the current CCCs will be evaluated in addition to applications from institutions not presently in the network. A separate solicitation ( ) seeks limited applications for a DCC that will support the network.
Three mock cardiothoracic surgical studies (randomized controlled trial, proof-of-concept trial, and registry/observational study) are presented in the Research Plan section of this announcement. Each of these is to be used as a basis for providing the following application responses: description of applicable patient populations and demonstration of sufficient patient volumes to support participation in the trial or study; innovative strategies to identify, recruit, randomize and retain large numbers of patients from broad patient groups; approaches for training of center staff; innovative ways to ensure compliance with all protocol requirements, and any other approaches to successfully complete network trials. Applicants must also describe their actual performance in other previous CTSN trials or non-Network cardiothoracic surgical trials.
Background
Cardiovascular disease (CVD) constitutes a large morbidity and mortality burden in the United States, and consumes a large portion of the health care bill. According to disease statistics in 2007, CVD caused 814,000 deaths, or 34 percent of all deaths. This cost the nation $286 billion in healthcare expenditures including $167 billion in direct health expenditures and $119 billion in indirect cost of mortality (source: NHLBI Disease Statistics). Improved medical and surgical management in the past 30 years has produced a decline in the death rate due to cardiovascular heart disease. Cardiothoracic (CT) surgery has been an important therapeutic tool in addressing many of the most prominent cardiovascular conditions, including coronary artery disease, congestive heart failure and valvular disease, among others. CT surgery has been very innovative in addressing these disease challenges. Coronary artery bypass grafting (CABG), cardiac valve repairs, ventricular assist devices, artificial hearts, and arrhythmia surgery have extended survival and improved the quality of life for many patients. However, the accelerating pace of innovation in CT surgical practice, including the utilization of new approaches and technologies and the extension of surgical approaches to older patients with greater co-morbidities, has outstripped the ability of randomized clinical trials to provide an evidence base to establish improved outcomes and benefit. In fact, the 2009 Initial National Priorities for Comparative Effectiveness Research by the Institute of Medicine reveals that more than half of all medical treatments delivered today are without clear evidence of effectiveness, including a top priority to compare the effectiveness of treatment strategies for atrial fibrillation via surgery, catheter ablation, and pharmacologic treatment, as well as a need to compare robotic vs. non-robotic surgical strategies. More recently, the NHLBI/AATS Cardiothoracic Surgery Symposium and Workshop (April 2011), endorsed the continuation of the current CTSN trials and support for launching pipeline trials, suggested the Network could be instrumental in optimizing long-term follow up of surgical patients, and recognized the Network as an ideal interdisciplinary infrastructure to link cardiothoracic surgeons with other medical research specialties.
The mandate of the Institute of Circulatory and Respiratory Health’s (ICRH) of the Canadian Institutes for Health Research amplifies the need for a growing number of clinician scientists and clinicians to contribute to the global health enterprise. There are approximately 140 cardiac surgeons in Canada. Those cardiac surgeons who also conduct research require adequate time, funding, mentoring, access to facilities and technology, and linkages with accomplished collaborators. The CTSN is an effective model for bringing together cardiac surgeons with many other health professionals and researchers to design new clinical trials that will speed the transfer of new knowledge into clinical practice.
The CTSN also aligns with at least two key priorities within ICRH’s recently updated strategic plan. In 2011, the ICRH of the CIHR identified the development of research networks, and the development of patient oriented research/knowledge transfer as two key strategic priorities. In fact, ICRH was an enabling force in developing the Canadian Institutes of Health Research - Strategy for Patient-Oriented Research, as well as contributing to CIHR's position on research networks. Working together with many partners, CIHR aims to transform Canada's capacity for patient-oriented research by: developing and sustaining the next generation of clinician-researchers, improving the infrastructure and funding for patient-oriented research, and supporting the development of relevant value-added research networks. This Network complements CIHR’s focus on development of patient-oriented research teams, platforms, networks and centers.
Currently, the CTSN consists of one data coordinating center (DCC), eight CCCs, two clinical skills development cores, four clinical core laboratories, a specimen biorepository, eight ancillary sites (which have an established collaborative relationship to CCCs) and eight satellite sites (which have no relationship to the CCCs, and are subcontracted through the DCC). Another ten satellite sites will be activated by February, 2012.
Ongoing Clinical Trials
The clinical research portfolio for the initial CTSN grant period falls into three areas: randomized clinical trials, observational prospective studies, and proof-of-concept trials.
Randomized Clinical Trials
The following trials are active and anticipated to complete randomization during the current grant period:
1. Surgical Interventions (CABG vs. CABG plus mitral valve repair) for Moderate Ischemic Mitral Regurgitation (MMR).
2. Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation (SMR).
3. Surgical Ablation Versus No Surgical Ablation for Patients with Persistent or Longstanding Persistent Atrial Fibrillation Undergoing Mitral Valve Surgery (AF).
Observational Prospective Studies
The following studies are completed:
1. Management Practices and the Risk of Infections Following Cardiac Surgery (Infections Registry).
2. Discrete Choice Study to Evaluate Patient Preference for Surgical vs. Non-surgical Procedures in Patients Undergoing Coronary Revascularization.
Proof-of-Concept Trials
The following trial is being conducted as a collaboration with the Cardiovascular Cell Therapy Research Network (CCTRN) and leverages both intellectual and infrastructure resources from the two Networks to accelerate the translation of a novel off the shelf product for heart failure patients. This CTSN-CCTRN collaboration is designed as a two-stage trial with Stage 1 randomization to begin in the current grant period. Provided the results of Stage 1 show that the protocol is feasible and the intervention is safe, Stage 2 will be conducted during the second grant period.
LVAD Therapy: Exploring the Effect of Intramyocardial Injection of Mesenchymal Precursor Cells on Myocardial Function (LVAD-MPC) - Stage 1
Specific Areas of Research Interest and Experimental Approaches Being Sought
The primary goal of the CTSN is to conduct multiple, collaborative, randomized clinical trials, proof-of-concept trials, and observational prospective clinical studies to evaluate surgical interventions and related management approaches to improve the treatment of cardiovascular disease and conditions in adult populations. Also of interest are strategies to reduce neurological complications, such as stroke, and optimize long-term cognitive and functional outcomes after CT surgery by evaluating neuroprotective approaches. Secondary objectives of the Network are to:
- Develop a collaborative infrastructure for this research.
- Promulgate the use of evidence-based medicine in CT surgical practice and related areas of cardiology.
- Inform the use of new interventions in surgical practice.
- Disseminate findings to the medical community.
- Train investigators in cardiothoracic surgical clinical research.
The CTSN emphasizes small translational, proof of concept and Phase 1 and 2 randomized trials and investigations. Phase 3 trials are outside the scope of this program. The Network is expected to manage multiple trials concurrently, and complete four to six Phase 1/2 trials and/or observational studies during the 5-year funding period. Potential research protocols might include, but are not limited to:
- LVAD Therapy: Exploring the Effect of Intramyocardial Injection of Mesenchymal Precursor Cells on Myocardial Function (LVAD-MPC) - Stage 2.
- Effect of Adding the Adenosine Diphosphate Receptor Inhibitor, Ticagrelor, to Aspiring on Major Adverse Cardiac and Cerebral Events after CABG Surgery.
- Trial Comparing Hybrid Revascularization Procedures to Multi-vessel PCI with Drug-eluting Stents in Patients with Multi-vessel CAD.
- Long-term follow-up of current CTSN MMR, SMR and AF trials.
- Trial to Evaluate the Effectiveness and Safety of Endovascular Stent Graft Treatment in Patients with Acute, Uncomplicated Type B, Aortic Dissection at High Risk for Complications.
- Surgical aortic valve replacement vs optimal medical management for asymptomatic aortic stenosis.
- Tricuspid annuloplasty vs. no annuloplasty in patients undergoing mitral valve surgery.
- Perioperative anticoagulation management in cardiac surgical patients.
- Surgical- vs. catheter-based (or combination) ablative strategies for atrial fibrillation.
- Test neuroprotective agents and novel management strategies, and validate current modalities for assessing neurologic injury and function in the context of major heart surgery.
- Observational registry of new-onset post-operative atrial fibrilation to inform future investigations.
Network Organization
The CTSN is a cooperative Network of academic Clinical Centers, a DCC, a rotating Network Chair (cardiothoracic surgeon) and rotating Co-Chair (cardiologist), and NHLBI, NINDS and ICRH Project Scientists.
Core Clinical Centers (CCCs) are responsible for proposing and developing protocols, recruiting study subjects, entering data into the web-based data capture system, assuring good clinical practice, mentoring junior investigators, and disseminating research findings. All CCCs are required to participate in a cooperative and interactive manner with one another and with the DCC and to enroll into all CTSN sponsored trials and studies. Up to eight CCCs will be funded through RFA HL-13-017, and up to 30 clinical consortium centers will be subcontracted through the DCC to enhance trial enrollment, protocol development, manuscript preparation, and other key Network activities.
The Data Coordinating Center (DCC) coordinates, administers, and supports all Network clinical research, operational, and administrative and statistical activities. Briefly, these activities include but are not limited to supporting protocol development; developing manuals of procedures and electronic case report forms; providing sample size calculations, statistical advice, common questionnaires, and data analysis; supporting manuscript preparation; and providing overall study coordination and quality assurance, including support for the Data and Safety Monitoring Board (DSMB), the Protocol Review Committee (PRC), the Steering Committee, and other standing committees meetings/conference calls. Funds to support execution of the protocols at the clinical centers are part of the DCC cooperative agreement (hereafter referred to as award ) and are distributed to the clinical centers by the DCC on a per patient basis and according to the approved protocol budgets. The DCC identifies and subcontracts with clinical consortium centers, core laboratories and biorepositories.
The Steering Committee (SC) is the main governing body of the CTSN. The SC comprises the Principal Investigators (PIs) from the CCCs and the DCC, the Network Chairs, NIH and CIHR staff. Voting members of the SC include the Chair (a cardiothoracic surgeon) and Co-Chair (a cardiologist), the PIs of the CCCs and DCC, and the NHLBI Program Official. The SC has primary responsibility for the general organization of the CTSN, the approval of clinical protocols and protocol changes, the conduct and monitoring of studies, and the expeditious reporting of study results. All major scientific and administrative decisions are determined by majority vote of the SC, which meets in person an average of twice a year and by teleconference on a monthly basis. This Committee ensures that at all decisions are reported to the Investigators Committee.
Committees and Subcommittees of the SC, such as the Operations Committee and subcommittees for Protocol Development, Protocol Operations, Publications and Biorepository have been established and may be continued or added to at the discretion of the SC.
The Investigators Committee (IC) consists of all CTSN investigators and study coordinators from the core clinical and clinical consortium centers, staff members from the DCC and NIH, and the CTSN Chairs. The Network Chair and Co-Chair are named by NHLBI to oversee and guide SC and IC activities. The IC meets in person an average of twice a year and by teleconference on a monthly basis. Subcommittees are established as necessary and membership includes physician and nurse investigators from the CCCs, representatives from the DCC, and members from NIH.
NHLBI is responsible for organizing and providing overall support for the CTSN. The NHLBI Program Office and Office of Grants Management are responsible for the federal stewardship of the award (management, financial and administrative oversight). In addition to regular award oversight, the NHLBI Project Scientists will be involved substantially with the awardees as a partner, consistent with the Cooperative Agreement mechanism. The NHLBI will appoint the Protocol Review Committee, the Data Safety and Monitoring Board, and the Network Chair and Co-Chair. The Study Chairs are independent of the DCC and CCCs and are responsible for ensuring that there are well-documented policies and procedures in place to guide all aspects of Network activities and operation. In collaboration with NHLBI staff the Chairs facilitate Network activities; oversee its functions; and conduct SC and IC meetings.
ICRH will provide and administer a portion of the protocol funds for the CCC awarded to a successful academic institution located in Canada. ICRH staff will work with NHLBI to assess responsiveness, collaborate with the NHLBI concerning the peer review process and administration, and advise the NHLBI on matters related to the Network.
An independent Protocol Review Committee (PRC) is appointed by and advisory to the NHLBI in consultation with ICRH. It consists of a chairperson and clinicians and scientists with expertise in basic and clinical cardiothoracic surgery research, clinical trial design, biostatistics, enabling technologies, outcome measures, and other areas of expertise as needed. The PRC will evaluate protocols approved by the SC based on the following criteria: importance of the question to be addressed, the scientific merit of the experimental design and approach, feasibility, appropriateness for the Network, and consistency with NHLBI missions and policies. All new study protocols performed by the CTSN must be approved by the PRC before referral to the DSMB. The PRC may be copied on minor protocol amendments or low-burden ancillary studies.
An independent Data and Safety Monitoring Board (DSMB) is appointed by and advisory to the NHLBI in consultation with the ICRH, in accordance with established policies to ensure data quality and participant safety. All network protocols must be approved and monitored by the DSMB, generally after their approval by the PRC (except in cases of minor protocol amendments, low-burden ancillary studies or secondary analyses of approved trials). The DSMB will be responsible for providing independent advice to the NHLBI regarding the progress of each trial and the appropriateness of continuing each study. The DSMB will meet approximately every six months, with interim meetings as necessary.
An independent External Review Committee with expertise in areas such as cardiothoracic surgery, cardiology, cellular therapy, as well as clinical trial management and regulatory oversight will review the CTSN during the third year of the network and advise the NHLBI (and SC) on opportunities to improve operations and future scientific directions.
Scientific and Performance Requirements for the Core Clinical Centers (CCC)
CCCs will be evaluated on the strength of their scientific application for innovative trial operations as well as their record of success supporting previous cardiothoracic surgical clinical trials, especially with regard to proper trial conduct and meeting enrollment targets.
CCCs propose and develop network protocols; screen, identify and recruit patients; and disseminate research findings. All individual CCCs are required to participate in a cooperative and interactive manner with one another and with the DCC, and enroll in all trials of the CTSN. Thus, CCC applicants must have demonstrable experience conducting complex multi-center clinical trials and studies in cardiothoracic (CT) surgery and a history of previous successful clinical research. CCC applicants must demonstrate excellence in clinical research to perform CT surgical interventions on the range of cardiovascular diseases and conditions that the network may address, and a proven ability to recruit patients with these diseases and conditions from various racial/ethnic groups into clinical trials of the type that will be done in this network. Prospective CCCs must have demonstrated access to a sufficient number of patients to accomplish their portion of the proposed protocols (approx. 1/8 of the total). The latter requirement includes demonstrating access to sufficient numbers of eligible patients and might necessitate forming partnerships with local primary care practice groups and hospitals. A CCC application will be considered non-responsive to this FOA if there are fewer than 500 major cardiac surgical procedures performed each year at the CCC. Potential applicants should review the additional information contained in Section IV, Application Submission Instructions.
The PDs/PIs of the CCC are expected to have demonstrated scientific leadership in the conduct of CT surgical clinical trials and studies. The PDs/PIs are responsible for proposing and participating in protocol development, estimating trial costs, conducting the clinical trial and study activities, assuring quality of patient care and protocol adherence, assuring the accurate and timely transmission of data to the DCC and core laboratories, and collaborating with other CTSN investigators in the publication and dissemination of research findings.
The CCC is subject to annual administrative review. Also, the entire CTSN program, including the DCC and CCCs, will be reviewed by an External Review Committee during the third year.
Funding Instrument |
Cooperative Agreement |
Application Types Allowed |
New The OER Glossary and the PHS398 Application Guide provide details on these application types. |
Funds Available and Anticipated Number of Awards |
The number of awards is contingent upon NIH appropriations, and the submission of a sufficient number of meritorious applications. Up to 8 CCCs and 3 Clinical Research Skills Program awards will be funded under this FOA. The total amount of funding (direct and indirect costs) that will be available through this announcement is up to $21,700,000 over a five-year period to support the CTSN. The following NIH components intend to commit the
following amounts in FY2013: ICRH intends to commit a maximum of $300,000 toward a meritorious application from a Canadian institution. ICRH will consider allocating funding toward both protocol costs and a Clinical Research Skills Development Program. Applicants who wish to have their projects considered for funding by ICRH should include with their application a letter stating that their application and summary statement may be shared with ICRH. |
Award Budget |
Each CCC may request annual direct costs up to $250,000. CCCs proposing a Clinical Research Skills Development Program may budget an additional $100,000 direct costs per year. |
Award Project Period |
Five years |
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made in response to this FOA.
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
- Eligible Agencies of the Federal Government
- U.S. Territory or Possession
Other
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Non-domestic (non-U.S.) Entities (Foreign Institutions) are eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement, are allowed.
Public research organizations already recognized for funding by the Canadian Institutes for Health Research and listed as such on the CIHR website.
Applicant organizations must complete the following registrations as described in the PHS398 Application Guide to be eligible to apply for or receive an award. Applicants must have a valid Dun and Bradstreet Universal Numbering System (DUNS) number in order to begin each of the following registrations.
- Central Contractor Registration (CCR) must maintain an active registration, to be renewed at least annually.
- eRA Commons
All Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) must also work with their institutional officials to register with the eRA Commons or ensure their existing eRA Commons account is affiliated with the eRA Commons account of the applicant organization.
All registrations must be completed by the application due date. Applicant organizations are strongly encouraged to start the registration process at least4-6 weeks prior to the application due date.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from underrepresented racial and ethnic groups as well as individuals with disabilities are always encouraged to apply for NIH support.
This FOA does not require cost sharing as defined in the NIH Grants Policy Statement.
Applicant organizations may submit only one application.
Applicants are required to prepare applications according to the current PHS 398 application forms in accordance with the PHS 398 Application Guide.
It is critical that applicants follow the instructions in the PHS398 Application Guide, except where instructed in this funding opportunity announcement to do otherwise. Conformance to the requirements in the Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows IC staff to estimate the potential review workload and plan the review.
By the date listed in Part 1. Overview Information, prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed research
- Name, address, and telephone number of the PD(s)/PI(s)
- Names of other key personnel
- Participating institutions
- Number and title of this funding opportunity
The letter of intent should be sent to:
Director, Office of Scientific Review
Division of Extramural Research Activities
National Heart, Lung, and Blood Institute
National Institutes of Health
6701 Rockledge Drive, Room 7214, MSC 7924
Bethesda, MD 20892-7924 (Express: 20817)
Telephone: (301) 435-0270
FAX: (301) 480-0730
Email: [email protected]
Applications must be prepared using the PHS 398 research
grant application forms and instructions for preparing a research grant
application. Submit a signed, typewritten original of the application,
including the checklist, and three signed photocopies in one package to:
Center for Scientific Review
National Institutes of Health
6701 Rockledge Drive, Room 1040, MSC 7710
Bethesda, MD 20892-7710 (U.S. Postal Service Express or regular mail)
Bethesda, MD 20817 (for express/courier service; non-USPS service)
At the time of submission, two additional paper copies of
the application and all copies of the Appendix files must be sent to:
Director, Office of Scientific Review
Division of Extramural Research Activities
National Heart, Lung, and Blood Institute
National Institutes of Health
6701 Rockledge Drive, Room 7214, MSC 7924
Bethesda, MD 20892-7924 (Express: 20817)
Telephone: (301) 435-0270
FAX: (301) 480-0730
Email: [email protected]
All page limitations described in the PHS398 Application Guide and the Table of Page Limits must be followed, with the following requirement:
- Optional Clinical Research Skills Development Program Section limited to 6 pages.
- Research Strategy section is limited to 30 pages.
All instructions in the PHS398 Application Guide must be followed, with the following additional instructions:
Surgical trials are complex and challenging to conduct, and the CSTN has not been immune to such difficulties. Thus, in this phase, it will be important to adopt new strategies for patient recruitment, retention and logistical and regulatory methodology to ensure successful and timely completion of the network trials.
Examples of such patient recruitment strategies could include processes to ensure clinical equipoise among CCC investigators, tailored recruitment targeted at desired patient populations which may necessitate approaches in addition to referrals such as, multimedia outreach, publicizing trials via patient-advocacy groups, and using social media sites to identify and engage trial participation. Patient retention strategies could include, but are not limited to, more frequent follow-ups, establishing satellite/mobile sites to increase convenience to patients, personal recognitions of appreciation, keeping participants informed of trial progress, and involving participants in appropriate study committees as well as celebrations of trial milestones. Logistical and regulatory methodology could include use of centralized IRBs and creative Informed Consent templates, among other strategies.
Three mock cardiothoracic surgical studies are presented which are representative of CTSN trials. Within the Research Plan and for each of the three mock trials, applicants should include a description of patient populations; innovative strategies for outreach, identification of qualified patients, recruitment, randomization and retention of large numbers of patients from broad patient groups; as well as approaches to train center staff, ensure compliance with all protocol requirements, and any other approaches to successfully complete network trials. In addition, CCC applicants should identify areas or issues that may present difficulties, and propose innovative strategies to overcome them for each trial. Responses are limited to 3 pages for each mock trial.
Study 1 - Randomized Clinical Trial - Effectiveness of Surgical Mitral Valve Repair and Optimal Medical Management Versus Optimal Medical Management Alone for Patients With Significant Mitral Regurgitation and Non-Ischemic Congestive Heart Failure (EXAMPLE-RCT)
This is an example of an attempted trial in a Network setting which was closed early due to recruitment challenges. Surgical mitral valve repair for secondary mitral regurgitation (MR) is controversial. Whether addition of corrective surgery to mechanically reduce MR adds benefit to optimal medical therapy (OMT) alone in dilated cardiomyopathy patients is unknown. EXAMPLE-RCT will be a randomized, controlled, multi-center trial to evaluate the safety and efficacy of mitral repair plus optimal medical management (OMM) versus OMM alone in patients with significant mitral regurgitation (MR) and non-ischemic congestive heart failure (CHF). Patients (n=120) with symptomatic chronic CHF (NYHA Class II-IIIb), documented left ventricular ejection fraction (LVEF) < 0.35, significant MR without primary underlying structural abnormalities of the mitral apparatus, and non-significant coronary disease will be recruited over an 18-month period. Consented patients will enter a 4-week run-in period during which they will undergo transesophageal echocardiography (TEE) and cardiopulmonary exercise testing (CPX). Those patients with moderate to severe secondary MR and peak oxygen consumption (VO2) < 22 mL/kg/min will be randomized 1:1 to mitral repair plus OMM or OMM alone. Baseline clinical assessment, quality of life questionnaire, formal transthoracic echocardiogram (TTE) to assess LV geometry, and neurohormonal and inflammatory biomarker evaluation will occur at randomization. At 1, 3, 6, 12, and 18 months post-randomization, all patients will undergo repeat evaluation with clinical assessment, quality of life questionnaire, neurocognitive status testing, TTE, CPX, and biomarker collection. Formal follow-up will be completed at 18 months. Long-term mortality follow up out to 5 years will be performed using the National Death Index. The primary end-point assessing LV remodeling will compare the difference from baseline to 18 months between arms using intention-to-treat analysis for LV end-systolic volume index (LVESVI).
Study 2 - Observational Study - Aortic Valve Disease Registry (EXAMPLE-Registry)
The purpose of this prospective multi-center, observational registry is to obtain clinical baseline, procedural and follow-up data on patients with aortic valve disease, as well as to assess clinical (short, mid- and long-term) outcome data. Specific objectives include:
- Description of structure, process, and outcome quality for the various aortic valve therapies.
- Definition of indication criteria (e.g., through scoring systems).
- Collection of safety and efficacy data.
- Collection of quality of life data prior to and after treatment.
- Health economic evaluation of the applied treatments.
- Informing future clinical trials.
Approximately 5,000 patients diagnosed with aortic valve insufficiency and/or stenosis who receive one of the following interventions will be recruited:
- Surgical aortic valve replacement.
- Aortic valve surgery (Ross or David/Modified David procedure).
- Transcatheter aortic valve implantation (transvascular or transapical).
- Aortic valve valvuloplasty as the principal indication for concurrent combination procedures (e.g., mitral valve surgery, coronary artery bypass grafting).
Each participating site will collect baseline and treatment data as well as outcome data at time of diagnosis, 30 days, 6 months and thereafter every 6 months for up to 5 years.
Primary outcome measures are all cause mortality at:
- 30 days
- 6 months
- 1 year
- 3 years
- 5 years
Secondary outcome measures are:
Non-fatal severe complications, including aortic valve re-intervention, coronary bypass surgery, myocardial infarction, stroke, other thromboembolic events, severe bleeding requiring transfusion, PCI, ICD or pacemaker placement, and/or dialysis
- during hospitalization
- 30 days
- 6 months
- 1 year
Severity of heart failure assessment using NYHA-classification
- 30 days
- 1 year
- 3 years
- 5 years
Quality of Life assessment (EuroQoL 5D Questionaire) preoperatively by personal interview and post-operatively by telephone interview
- 1 year
- 3 years
- 5 years
Study 3 - Early phase trial - A Dose Escalation and Safety Trial of Difungomuctane (XYZ-1234) in Patients Undergoing Major Cardiothoracic Surgery (EXAMPLE-Early Phase)
The fictitious drug difungomuctane (XYZ-1234) is a small molecule that is being developed to protect patients from acute brain injury after cardiac bypass surgery. This first-in-man study will be a phase 1, multi-center, randomized, double-blind, dose escalation, safety and pharmacokinetic trial of a single intravenous injection of difungomuctane in patients undergoing major cardiothoracic surgery. Up to 45 patients who will undergo non-emergent coronary artery bypass graft and/or valve replacement surgery and require a cardiopulmonary bypass machine between the ages of 21 and 85 years will be recruited. Patients will receive a single intravenous injection of difungomuctane or saline control in a 2:1 randomization 4 hours + 30 minutes following removal of the cardiopulmonary bypass machine according to the following schedule:
Dose Escalation Schedule |
|
Dose Level |
Dose of Difungomutance |
Level 1 |
30 mcg/kg* |
Level 2 |
60 mcg/kg |
Level 3 |
90 mcg/kg |
Level 4 |
120 mcg/kg |
Level 5 |
180 mcg/kg |
*1/6 maximally tolerated dose in dogs. |
|
The initial dose will be 30 mg/kg (1/6 the maximal tolerated
dose in dogs). Dose escalation will proceed within each cohort according to
the following scheme:
Number of Patients with DLT* at a Given Dose Level |
Escalation Decision Rule |
0 out of 3 |
Enter 3 patients at the next dose level |
> 2 |
Dose escalation will be stopped. This dose level will be declared the maximally administered dose (highest dose administered). Three (3) additional patients will be entered at the next lowest dose level if only 3 patients were treated previously at that dose. |
1 out of 3 |
Enter at least 3 more patients at this dose level. If 0 of these 3 patients experience DLT, proceed to the next dose level. If 1 or more of this group suffer DLT, then dose escalation is stopped, and this dose is declared the maximally administered dose. Three (3) additional patients will be entered at the next lowest dose level if only 3 patients were treated previously at that dose. |
<1 out of 6 at highest dose level below the maximally administered dose |
This is generally the recommended phase 2 dose. At least 6 patients must be entered at the recommended phase 2 dose. |
* DLT: Dose Limiting Toxicity
The duration of the study is approximately 74 days, inclusive of a 14-day screening period. Patient evaluation visits will occur as follows: screening; day of surgery; hospital in-patient Days 1, 2, 3, and 7 or hospital discharge (whichever comes first); 30 and 60 days post-surgery. Patients will be contacted by phone at 6 and 12 months post-surgery for follow-up questions.
Primary outcome measures are:
Number of dose-limiting toxicities i.e., observed among up to 5 cohorts of 3-6 patients per cohort; dose-limiting toxicities are defined as:
- Drug-related severe or life-threatening non-hematological toxicity
- Life-threatening hematological toxicity >4 days
- Severe diarrhea >4 days despite aggressive management
- Severe vomiting >24 hours despite suitable anti-emetics
Pharmacokinetics: immediately following injection through 24 hours.
General Requirements for the CCC
The Network is a collaborative effort requiring all CCCs to participate in a cooperative and interactive manner with all components. Applicants should explicitly indicate their willingness to:
- Facilitate and participate in SC meetings, site visits, and regular telephone conference calls.
- Cooperate with other awardees in the development and design of research protocols.
- Promote and abide by common definitions, common methods for patient selection and enrollment, and common protocols, procedures, tests, and reporting forms as chosen by majority vote of the SC.
- Actively implement each network-wide protocol approved by the NHLBI-appointed PRC and NHLBI.
- Comply with study policies and quality assurance measures approved by the SC.
- Agree to oversight of the study by the NHLBI-appointed DSMB.
- Transmit study data to the DCC and NHLBI in a timely and accurate manner.
- Report all adverse events in accordance with procedures established by the SC, NHLBI policies and other applicable federal policies.
- Cooperate with other awardees in the publication of study results and the eventual release to the scientific community of study procedures and other resources.
- Participate in studies of the cost effectiveness of therapeutic interventions should the NHLBI choose to implement such research within the Network.
- Accept the Cooperative Agreement Terms and Conditions of Award which are found in Section VI, Award Administration Information.
Within the Research Plan, CCC applicants should submit verified benchmark data from all cardiothoracic surgical trials conducted at the site during the past five years. Benchmark data should include information (compared to the overall trial average) on: 1) the number of patients recruited and site-specific accrual targets for each trial, 2) timeliness of enrollment (time from site activation to first enrollment), 3) endpoint completion (submission of primary and secondary endpoint data), 4) patient retention (subjects completing final study visit, and 5) data quality and timeliness. Tabular presentation of such data is preferred and an example template is given below.
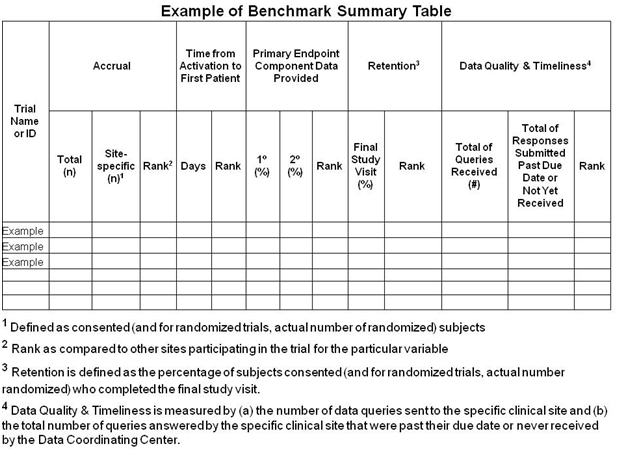
All benchmark data submitted should be verified as much as possible by the DCC of the trial in the form of a letter included in the application package. The verification letter should appear in the application as a letter of support and is not part of the 30 page limit.
Additionally, departmental and institutional commitment to supporting cardiothoracic surgery trials and studies and to prioritization of Network trials and studies should be clearly documented by the leadership in the form of letters or memoranda provided to the PI, and by citing evidence of past support. These assurances to provide support should address areas such as fiscal administration, personnel management, space allocation, procurement, planning and equipment.
Clinical Research Skills Development Programs
The CTSN has the potential to provide hands-on surgical research experience for residents and junior faculty investigators. The goals of the Clinical Skills Development Program are to motivate and equip future cardiothoracic surgeons to pursue a career involving research and clinical trials. The objectives are to provide future cardiothoracic surgeons with the necessary didactic preparation and research-intensive experiences within a closely mentored framework.
CCC applicants are not required to submit an application for a Clinical Research Skills Development Program, and it will have no effect on the overall score of the CCC application. Applicants may request up to $100,000 per year in direct costs for a Clinical Research Skills Development Program, and not more than two programs will be funded by the NHLBI in the CTSN each year.
Applicants should describe the clinical research mentoring needs or gaps for cardiothoracic surgeons/researchers, at what stage in their professional career this activity should take place, and how the proposed program will establish activities to bridge the gap. Skills development programs are expected to include a combination of mentoring, didactic coursework and research-intensive experiences involving CTSN trials and studies. Examples include direct participation in various components of the trials (e.g., case report form screening, triaging, recruitment, informed consent, patient logistics, and follow-up care, etc.) as well as analyses of studies from related cardiothoracic surgical experiences.
Because the funds available for the skills development program are limited, opportunities to partner with professional societies, foundations, private sources and/or leverage other resources at the CCC institution and affiliated sites, CTSA infrastructure, as well as distance-learning opportunities should be presented. Mentored and innovative cross-disciplinary research experiences are encouraged to introduce new research techniques, technologies and opportunities relevant to CTSN trials.
The skills development programs are intended for residents and investigators with limited clinical research experience. Candidates for the clinical research skills development program can include residents and junior faculty, however, investigators with previous or current K-series awards cannot participate in the CTSN skills development program.
Leaders for these programs must be identified and should have strong educational and mentoring credentials and be able to contribute a minimum of 0.6 calendar months). To facilitate mentoring and multidisciplinary developmental activities, active involvement by the PI and other senior investigators within the CTSN is strongly encouraged.
Clinical Research Skills Development Program applications will be evaluated on the basis of how well they address the following elements:
- Identification of the most important deficiencies in the preparation of residents and junior faculty for pursuing a career in cardiothoracic surgery research.
- A summary of the types of skills that would be developed and a description of proposed project-specific activities.
- Details of how mentoring and the professional development of the new clinical investigators will be achieved, including their progression to more independent status.
- A plan for recruiting high quality participants, including plans to recruit women and minorities.
- The credentials and experience of the program leaders and other participating senior staff.
- A plan for coordinating the activities and progress of participants.
- A description of related activities within the applicant's institution and affiliated organizations, and steps taken to leverage them and avoid overlap and duplication.
- Describe any partnerships with professional societies or foundations that may provide stipend or other support, or leveraging of related resources or infrastructure.
An application for a Clinical Research Skills Development Program will be evaluated in terms of its potential effectiveness in developing the skills and research capabilities of new clinical investigators as reflected in the required application components identified above. Scoring for this component is separate and distinct from scoring for the CCC application.
The scientific and performance requirements must follow the requirements listed above and the instructions in the PHS 398 application Continuation Format Page form http://grants.nih.gov/grants/funding/phs398/phs398.html#fp3. The total length of the Clinical Research Skills Development Program section should not exceed 6 pages. These pages will not be counted against the 30 page Research Strategy limit and should not be included in the Research Strategy Section of the application. Rather this optional section should be placed immediately before the Checklist in the application and pages must be numbered sequentially with the rest of the application. The presence of a Clinical Research Skills Development Program must be noted at the beginning of the Abstract.
Budget guidelines for the CCC
Applicants should prepare itemized (not modular) CCC budgets for five one-year periods with annual direct costs not to exceed $250,000 per year. The budget is expected to maintain the infrastructure required to perform multiple clinical trials. CCC budget costs must follow the PHS 398 instructions but must also include:
- A minimum of 0.1.2 person months for the surgical physician leadership Principal Investigator).
- A minimum of 3.6person months for a cardiologist or neurologist Co-Investigator.
- A minimum of 12 person months for a nurse coordinator.
- A minimum of 6 person months for data entry personnel.
- Travel for the Principal Investigator and Co-Investigator(s) to an average of 2 SC and 2 all-investigator meetings per year. Travel for a clinical coordinator to 2 SC meetings and an additional coordinators meeting per year. These meetings usually are held in Bethesda, Maryland.
During the grant cycle covered by this FOA, all protocol-associated patient costs will be awarded to the DCC, which will then be responsible for distributing funds to CCCs once per patient budgets are approved for each protocol and recruitment begins. These costs are not to be included in the CCC itemized budget.
Investigators involved in NHLBI Network clinical studies may find it advantageous to seek additional support from, or other involvement by, industry or other third parties (e.g., the VA, CMS, charitable foundations) for the studies themselves, for substudies, ancillary studies, and/or to supplement the Clinical Research Skills Development Program. Awardees will be expected to follow NHLBI policy concerning third party agreements if collaborations are established with Industry or other third parties. http://www.nhlbi.nih.gov/funding/policies/thirdparty.htm
Awards will be subject to administrative review annually. In addition, per the NHLBI trial accrual policy, all successful CCCs in collaboration with the DCC and NHLBI, will jointly establish enrollment targets and accrual plans in order to achieve trial goals. For studies in which overall recruitment falls below negotiated levels, corrective action plans may be imposed. NHLBI may close a study if further efforts are deemed unlikely to bring the study to completion within an acceptable budget or time frame. In addition, network sites which consistently fall below their individual enrollment targets run the risk of losing continued funding.
Applicants proposing Clinical Research Skills Development Programs may budget an additional $100,000 direct costs per year as a separate, additional line item in the budget. Note: this line item is in addition to the CCC maximum allowable direct costs (see above).
Each CCC must designate a research coordinator with adequate effort as described above. A description of this individual's training, experience and involvement in clinical research should be provided. Appropriate effort for other key personnel, should be included with appropriate justification.
It should be noted that funds will not be provided for the purchase of expensive medical equipment, such as echocardiographic, cardiopulmonary exercise testing, magnetic resonance imaging or ultrafiltration systems.
CTSN and NIH Clinical and Translational Award (CTSA) Organizations
The NHLBI encourages academic centers participating in the CTSN to partner with a CTSA, if one exists at the applicant institution, to enhance the scientific and operational aspects of the Network.
There are many potential ways that a CTSN CCC could interface with a CTSA structure. Specific examples include utilizing some of the educational and mentoring programs within the CTSA organization to enhance a Clinical Research Skills Development program. Since one of the goals of the CTSA network is to establish strong and interactive relationships with local communities and clinics, there may be potential for enhanced recruitment of clinically and ethnically diverse patient populations through the CTSA. CCCs might partner with translational investigators in the CTSA to create state-of-the art mechanistic and translational sub-studies that could be conducted within the Network’s clinical protocols.
Resource Sharing Plan
Individuals are required to comply with the instructions for the Resource
Sharing Plans (Data Sharing Plan, Sharing Model Organisms, and Genome Wide
Association Studies (GWAS)) as provided in the PHS398 Application Guide.
All applications, regardless of the amount of direct costs requested for any one year, should address a Data Sharing Plan and comply with NHLBI’s Policy for Data Sharing.
Appendix
Do not use the Appendix to circumvent page limits. Follow all instructions for the Appendix (please note all format requirements) as described in the PHS398 Application Guide
Foreign (non-US) Institutions must follow policies described in the NIH Grants Policy Statement, and procedures for foreign institutions described throughout the PHS398 Application Guide.
Part I. Overview Information contains information about Key Dates.
Information on the process of receipt and determining if
your application is considered on-time is described in detail in the PHS398 Application
Guide.
Applicants may track the status of the application in the eRA Commons, NIH’s electronic system for grants
administration.
This initiative is not subject to intergovernmental review.
All NIH awards are subject to the terms and conditions, cost
principles, and other considerations described in the NIH
Grants Policy Statement.
Pre-award costs are allowable only as described in the NIH
Grants Policy Statement.
Applications must be received on or before the due dates in Part I. Overview Information. If an
application is received after that date, it will not be reviewed.
Non-Responsive Applications
Upon receipt, applications will be evaluated for completeness by the Center for Scientific Review and responsiveness by components of participating organizations, NIH. Applications that are incomplete and/or nonresponsive will not be reviewed.
Responsiveness issues should be addressed in the application. A CCC application will be considered non-responsive if any of the following conditions apply:
- PI is not a cardiothoracic surgeon at 1.2 person months level of effort
- Absence of a cardiologist or neurologist investigator at a minimum of 3.6 person months level of effort
- Absence of a neurologist Investigator.
- More than one CCC application is submitted from a single institution.
- Fewer than 500 major cardiac surgical procedures are performed per year at the CCC site.
- Failure to include DCC-verified benchmark data for all cardiothoracic surgical trials at the CCC site in the last five years.
- Failure to include a Letter(s) of Departmental Collaboration and Support, that describes the department’s support and acknowledgement that CTSN clinical trials are to take the highest priority over studies/trials at the proposed CCC which may be supported by other sources. This letter must also describe the strategy and criteria for allocating patients to CTSN trials versus other potential competing studies/trials. The letter should appear in the application as a Letter of Support and is not a part of the 30 page limit.
This FOA is open to all applicants with experience in cardiothoracic surgical trials and studies, regardless of whether they have previously participated in CTSN clinical trials. CCC applicants must be at an institution with an existing cardiothoracic surgical research program and demonstrated access to a sufficient number of patients for CTSN protocols.
Regarding investigator qualifications, applicants should provide evidence of productivity and relevant past performance in previous or ongoing cardiothoracic surgical trials or studies including those with a cooperative or multicenter design. Applicants should indicate whenever applicable (1) where their participation in a single institution study led to the implementation of a multicenter study; (2) contributions in key areas of protocol development and design, accrual planning and monitoring, patient recruitment, retention and study completion, data collection and analysis; and (3) relevant publications.
Please note that a decision to fund a particular CCC does not necessarily commit the CTSN to implement the proposed recruitment and retention strategies from that CCC.
In addition to the Benchmark Table, applicants who are current CTSN CCCs should include a description of their participation and contribution to the Network, including:
- Explanation of recruitment targets and recruitment attainment.
- Screening approaches to identify subjects for CTSN trials within the applicant’s institution.
- Specific roles of the applicant(s) in CTSN trials (principal investigator, co-investigator, SC member, etc.).
- Other contributions to Network activities (standing subcommittees, leadership roles in SC, and publications).
In addition to the Benchmark Table, applicants who are not current CTSN CCCs should include a description of their recent experience in early translational, proof of concept, and Phase I/II cardiothoracic surgical clinical trials or studies under IND/IDE, and observational studies including:
- Explanation of recruitment targets and recruitment attainment.
- Screening approaches to identify subjects for clinical protocols within the applicant’s institution.
- Specific roles of the applicant(s) (e.g., principal investigator, participation in clinical trial design and development, analysis and dissemination of data).
- Number of centers involved and the proportion of patients enrolled from the applicant’s center in multicenter studies.
- Contributions to cooperative group or multi-site clinical trial activities, as appropriate.
R&R Budget Component
This FOA uses non-modular budget formats described in the PHS 398 application instructions (see http://grants.nih.gov/grants/funding/phs398/phs398.html).
All foreign applicants must complete and submit budget requests using the Research & Related Budget component found in the application package for this FOA. See NOT-OD-06-096.
Investigators must submit a complete, justified, individual budget for each year of support requested. All costs requested and all changes in budgets after the first year should be clearly identified and justified. Separate itemized budgets must be prepared for each subcontract and/or for each collaborating site or core, if multiple sites or cores are proposed. If parts of the costs of the trial are to be borne by sources other than NIH, these contributions must be presented in detail along with supporting letters signed by individuals who have the authority to make fiduciary commitments on behalf of the institution. These outsource costs do not constitute cost sharing as defined in the current NIH Grants Policy Statement and should not be presented either as part of the requested budget or as Estimated Project Funding.
Further information concerning budget preparation may be obtained from the Office of Grants Management contact listed in Section VII. 3. Financial/Grants Management Contact(s).
Applicants are required to follow the instructions for post-submission materials, as described in NOT-OD-10-115.
Only the review criteria described below will be considered
in the review process. As part of the NIH mission,
all applications submitted to the NIH in support of biomedical and behavioral
research are evaluated for scientific and technical merit through the NIH peer
review system.
Reviewers will provide an overall impact/priority score to reflect their assessment of the likelihood for the proposed recruitment/retention/logistical strategies to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the recruitment/retention/logistical strategies proposed).
Reviewers will consider each of the review criteria below in the determination of scientific merit, and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a proposed recruitment/retention/logistical strategy that by its nature is not innovative may be essential to advance a field.
Significance
Does the proposed recruitment/retention/logistical strategy address an important problem or a critical barrier to progress in the field? If the aims of the approach are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
Investigator(s)
Are the PD(s)/PI(s), Investigator, collaborators, and other researchers well suited to the conduct of cardiothoracic surgical trials? If Early Stage Investigators or New Investigators, or in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD(s)/PI(s), Do the investigators have complementary and integrated expertise; are their leadership approach, governance and organizational structure appropriate for the project?
In addition, for this FOA, the following review criteria will also be applied:
1. What evidence is provided of investigator success in carrying out cardiothoracic surgical clinical trials and studies (i.e., meeting study recruitment/retention goals, data completeness reports, producing peer-reviewed publications, etc.)?
2. What evidence is provided to demonstrate that the CCC staff has the expertise, training, and experience to conduct multiple protocols effectively in a Network?
3. How have the PD(s)/PI(s) and co-Investigator(s) demonstrated clinical equipoise in their approaches to trial recruitment and retention?
4. Does the research team include expertise in strategies for neuroprotection or pre/post-operative neurological assessment?
5. Is adequate medical and regulatory expertise involved?
Innovation
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Approach
Are the overall strategy, methodology, and analyses
well-reasoned and appropriate to accomplish the specific aims of the collaborative
clinical trials and studies? Are potential problems, alternative strategies,
and benchmarks for success presented? If the project is in the early stages of
development, will the strategy establish feasibility and will particularly
risky aspects be managed?
Since the project involves clinical research, are the plans for 1) protection
of human subjects from research risks, and 2) inclusion of minorities and
members of both sexes/genders, as well as the inclusion of children, justified
in terms of the scientific goals and research strategy proposed?
In addition, for this FOA, the following review criteria will also be applied:
- How does the research plan demonstrate understanding of the scientific, statistical, logistical, and technical issues underlying multi-center cardiothoracic surgical clinical trials, and include feasible alternative approaches?
- How does the CCC demonstrate an ability to participate in collaborative research and cooperate with a Network Steering Committee and the NHLBI?
Environment
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
In addition, for this FOA, the following review criterion will also be applied:
- How adequate are the CCC facilities, equipment, sponsorship and organizational structures for effectively implementing the CTSN study protocols? This includes, but is not limited to, conducting studies, obtaining study and investigational agents and devices, and meeting the recruitment requirements.
As applicable for the innovative recruitment/retention/logistical approaches proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact/priority score, but will not give separate scores for these items.
Review Considerations for Clinical Research Skills Development Programs
Applications for a Clinical Research Skills Development Program will receive an impact score based on the review criteria below, but the impact score will be separate from and will not enter into the overall CCC application impact score. The Clinical Research Skills Development Program will be evaluated for its effectiveness in developing the skills and clinical research capabilities of future cardiothoracic surgeons. This includes an evaluation of:
- The most important deficiencies in the preparation of residents and junior faculty for pursuing a career in cardiothoracic surgery research.
- A summary of the types of skills that would be developed and a description of proposed project-specific activities.
- Details of how mentoring and the professional development of the new clinical investigators will be achieved, including their progression to more independent status.
- A plan for recruiting high quality participants, including plans to recruit women and other underrepresented groups.
- The credentials and experience of the program leaders and other participating senior staff.
- A plan for coordinating the activities and progress of participants.
- A description of related activities within the applicant's institution and affiliated organizations, and steps taken to leverage them and avoid overlap and duplication.
- Partnerships and leveraging with professional societies or foundations that may provide stipend or other support.
- How effectively will the Clinical Research Skills Development program attract, equip and motivate future cardiothoracic surgeons for a career in clinical research?
Protections for Human Subjects
For research that involves human subjects but does
not involve one of the six categories of research that are exempt under 45 CFR
Part 46, the committee will evaluate the justification for involvement of human
subjects and the proposed protections from research risk relating to their
participation according to the following five review criteria: 1) risk to
subjects, 2) adequacy of protection against risks, 3) potential benefits to the
subjects and others, 4) importance of the knowledge to be gained, and 5) data
and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or
more of the six categories of research that are exempt under 45 CFR Part 46,
the committee will evaluate: 1) the justification for the exemption, 2) human
subjects involvement and characteristics, and 3) sources of materials. For
additional information on review of the Human Subjects section, please refer to
the Human
Subjects Protection and Inclusion Guidelines.
Inclusion of Women, Minorities, and Children
When the proposed approaches involve clinical research, the committee will evaluate the proposed plans for inclusion of minorities and members of both genders, as well as the inclusion of children. For additional information on review of the Inclusion section, please refer to the Human Subjects Protection and Inclusion Guidelines.
Vertebrate Animals
No vertebrate animal research will be included in this FOA.
Biohazards
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
Not Applicable
Renewals
For Renewals, the committee will consider the progress made in the last funding period.
Revisions
Not Applicable.
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact/priority score.
Applications from Foreign Organizations
Reviewers will assess whether the project presents special opportunities for furthering research programs through the use of unusual talent, resources, populations, or environmental conditions that exist in other countries and either are not readily available in the United States or augment existing U.S. resources.
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Resource Sharing Plans
Reviewers will comment on whether the following Resource Sharing Plans, or the rationale for not sharing the following types of resources, are reasonable: 1) Data Sharing Plan; 2) Sharing Model Organisms; and 3) Genome Wide Association Studies (GWAS).
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
Applications will be evaluated for scientific and technical merit by (an) appropriate NHLBI OSR Scientific Review Group(s), in accordance with NIH peer review policy and procedures, using the stated review criteria. Review assignments will be shown in the eRA Commons.
As part of the scientific peer review, all applications:
- May undergo a selection process in which only those applications deemed to have the highest scientific and technical merit (generally the top half of applications under review) will be discussed and assigned an overall impact/priority score.
- Will receive a written critique.
Appeals of initial peer review will not be accepted for applications submitted in response to this FOA.
Applications will be assigned on the basis of established PHS referral guidelines to the appropriate NIH Institute or Center and will compete for available funds with all other recommended applications submitted in response to this FOA. Following initial peer review, recommended applications will receive a second level of review by the NHLBI Advisory Council. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
After the peer review of the application is completed, the PD(s)/PI(s) will be able to access his or her Summary Statement (written critique) via the eRA Commons.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement.
If the application is under consideration for funding, NIH
will request "just-in-time" information from the applicant as
described in the NIH
Grants Policy Statement.
A formal notification in the form of a Notice of Award (NoA) will be provided
to the applicant organization for successful applications. The NoA signed by
the grants management officer is the authorizing document and will be sent via
email to the grantee’s business official.
Awardees must comply with any funding restrictions described in Section IV.5. Funding Restrictions. Selection
of an application for award is not an authorization to begin performance. Any
costs incurred before receipt of the NoA are at the recipient's risk. These
costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this FOA will be subject to the DUNS,
CCR Registration, and Transparency Act requirements as noted on the Award
Conditions and Information for NIH Grants website.
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Grantees, and Activities. More information is provided at Award Conditions and Information for NIH Grants. In addition, scientists supported by ICRH funds will comply with the policies and guidelines as outlined in the CIHR Grants and Awards Guide (http://www.cihr-irsc.gc.ca/e/805.html).
The following special terms of award are in addition to, and
not in lieu of, otherwise applicable OMB administrative guidelines, HHS grant
administration regulations at 45 CFR Parts 74 and 92 (Part 92 is applicable
when state and local governments are eligible to apply), and other HHS, PHS,
and NIH grant administration policies.
The administrative and funding instrument used for this program will be the
cooperative agreement an "assistance" mechanism (rather than an
"acquisition" mechanism), in which substantial NIH programmatic
involvement with the awardees is anticipated during the performance of the
activities. Under the cooperative agreement, the NIH purpose is to support and
stimulate the recipients' activities by involvement in and otherwise working
jointly with the award recipients in a partnership role; it is not to assume
direction, prime responsibility, or a dominant role in the activities.
Consistent with this concept, the dominant role and prime responsibility
resides with the awardees for the project as a whole, although specific tasks
and activities may be shared among the awardees and the NIH as defined below.
The
PD(s)/PI(s) will have the primary responsibility for:
The CCC PDs/PIs has the primary responsibility in all aspects
of CTSN studies, including proposing protocols, participating in their overall
development, preparing protocol budgets in collaboration with the DCC,
modifying proposals if indicated, recruiting study participants, conducting the
research, assuring quality of study participant care and protocol adherence,
assuring the accurate and timely transmission of data collected in conjunction
with the DCC, analyzing and interpreting data, preparing publications, and
working with the DCC and NHLBI to disseminate research findings. CCC PDs/PIs
will also be responsible for working with the DCC to develop common definitions
and standardization across protocols wherever appropriate. Awardees must agree
to the governance of the study through a SC.
Support or other involvement of industry or any other third party in the study -- e.g., participation by the third party; involvement of study resources or citing the name of the study or NHLBI support; or special access to study results, data, findings or resources -- may be advantageous and appropriate. However, except for licensing of patents or copyrights, or provision of routine goods and services within normal business operations that are ancillary to the operation of the research program, support or involvement of any third party will occur only following notification of and concurrence by NHLBI.
Study investigators are encouraged to publish and to release publicly and disseminate results and other products of the study in accordance with study protocols and governance. For applicable studies, data not previously released and other study materials or products not previously distributed are to be made available to individuals who are not study investigators, within three years of the end of the period of NHLBI support, provided such release is consistent with the study protocol and governance.
Awardees will retain custody of and have primary rights to the data and software developed under these awards, subject to Government rights of access consistent with current HHS, PHS, and NIH policies.
NIH Project Scientists will have substantial programmatic involvement that is above and beyond the normal stewardship role in awards, as described below:
The NHLBI Project Scientists will monitor patient recruitment and study progress, ensure disclosure of conflicts of interest, and ensure adherence to NHLBI policies. NHLBI will appoint the Network Chair and Co-Chair, all members of the Protocol Review Committee (PRC), and the Data and Safety Monitoring Board (DSMB). The Network Chair will be responsible for ensuring that there are well-documented policies, procedures, and bylaws to guide all aspects of Network activities and operations.
The NHLBI Project Scientists will serve on the SC and other study committees, when appropriate. The NHLBI and NINDS Project Scientists may work with awardees on issues coming before the SC and, as appropriate, other committees, e.g., recruitment, intervention, follow-up, quality control, adherence to protocol, assessment of problems affecting the study and possible changes in protocol, interim data and safety monitoring, final data analysis and interpretation, preparation of publications, and development of solutions to major problems such as insufficient participant enrollment (http://www.nhlbi.nih.gov/funding/policies/accrual_guidelines.htm).
The NHLBI reserves the right to phase-out or curtail the study (or an individual award) in the event of (a) failure to develop or implement a mutually agreeable collaborative protocol; (b) substantial shortfall in participant recruitment, follow-up, data reporting, or quality control; (c) major breach of the protocol or substantive changes in the agreed-upon protocol with which NHLBI cannot concur; (d) attaining of a major study endpoint before schedule with persuasive statistical significance; or (e) human subject ethical issues that may dictate a premature termination.
Additionally, an agency Program Official will be responsible for the normal scientific and programmatic stewardship of the award and will be named in the award notice.
Areas
of Joint Responsibility include:
Awardee(s) agree to the governance of the study through a SC.
The SC will have primary responsibility for the conduct of protocols and the
preparation of publications. SC voting membership shall consist of all PDs/PIs
(i.e., cooperative agreement awardees), one NHLBI Project Scientist, and the
Network Chair and Co-Chair. Each full member will have one vote. Awardee
members of the SC will be required to accept and implement policies approved by
the SC.
An independent Protocol Review Committee, established by the
NHLBI, will provide peer review for each network protocol. A Data and Safety
Monitoring Board will be appointed by the Director, NHLBI to provide overall
monitoring of interim data and safety issues. An NHLBI scientist, other than
the NHLBI Project Scientist, shall serve as Executive Secretary to the Boards.
Because the Boards serve as independent advisors to the NHLBI, study
investigators will not communicate with Board members regarding study issues,
except as authorized by the Board’s Executive Secretary.
Dispute Resolution:
Any disagreements that may arise in scientific or programmatic matters (within the scope of the award) between award recipients and the NIH may be brought to Dispute Resolution. A Dispute Resolution Panel composed of three members will be convened. It will have three members: a designee of the SC chosen without NIH staff voting, one NIH designee, and a third designee with expertise in the relevant area who is chosen by the other two; in the case of individual disagreement, the first member may be chosen by the individual awardee. This special dispute resolution procedure does not alter the awardee's right to appeal an adverse action that is otherwise appealable in accordance with PHS regulations 42 CFR Part 50, Subpart D and HHS regulations 45 CFR Part 16.
When multiple years are involved, awardees will be required to submit the Non-Competing Continuation Grant Progress Report (PHS 2590) annually and financial statements as required in the NIH Grants Policy Statement. Please note that Canadian Nominated Principal Applicant is required to submit a CIHR Progress Report entitled Progress Report for Long Term Grant Holders (5 or More Years) on an annual basis summarizing the outcomes and describing how the grant funds were used (http://www.cihr-irsc.gc.ca/e/797.html).
A final progress report, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement.
The Federal Funding Accountability and Transparency Act of 2006 (Transparency Act), includes a requirement for awardees of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All awardees of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over $25,000. See the NIH Grants Policy Statement for additional information on this reporting requirement.
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
GrantsInfo (Questions regarding application instructions and
process, finding NIH grant resources)
Telephone 301-710-0267
TTY 301-451-5936
Email: [email protected]
eRA Commons Help Desk(Questions regarding eRA Commons
registration, tracking application status, post submission issues)
Phone: 301-402-7469 or 866-504-9552 (Toll Free)
TTY: 301-451-5939
Email: [email protected]
NHLBI
Marissa A. Miller, D.V.M., M.P.H.
Division of Cardiovascular Sciences
National Heart, Lung, and Blood Institute
National Institutes of Health
6701 Rockledge Drive, Room 8214, MSC 7940
Bethesda, MD 20892-7940
Telephone: 301-594-1542
Email: [email protected]
ICRH
Jennifer Ralph
Assistant Director (Acting)
Institute of Circulatory and Respiratory Health
Canadian Institutes of Health Research (CIHR)
160 Elgin St., Ottawa ON K1A 0W9
Telephone: 613-941-0086
Fax: 613-954-1800
Email: [email protected]
Canada
Director, Office of Scientific Review
Division of Extramural Research Activities
National Heart, Lung, and Blood Institute
National Institutes of Health
6701 Rockledge Drive, Room 7214, MSC 7924
Bethesda, MD 20892-7924 (Express: 20817)
Telephone: (301) 435-0270
FAX: (301) 480-0730
Email: [email protected]
Ms. Tamara Kees
Grants Management Specialist, Office of Grants Management
Division of Extramural Research Activities
National Heart, Lung, and Blood Institute
National Institutes of Health
6701 Rockledge Drive, Rm 7174, MSC 7926
Bethesda, MD 20892-7926
Telephone: 301-435-0169
Email: [email protected]
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts. All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement.
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 45 CFR Parts 74 and 92.

