G.220 - R&R Other Project Information Form
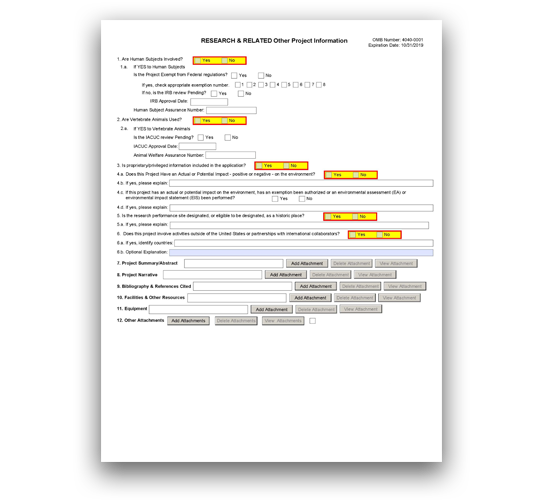
The R&R Other Project Information Form is used for all grant applications. This form includes questions on the use of human subjects, vertebrate animals, and environmental impact. This form also has fields to upload an abstract, project narrative, references, information on facilities, and equipment lists.
1a. If YES to Human Subjects
2. Are Vertebrate Animals Used?
2a. If YES to Vertebrate Animals
3. Is proprietary/privileged information included in the application?
4. Environmental Questions
5. Is the research performance site designated, or eligible to be designated, as a historic place?
6. Does this project involve activities outside of the United States or partnerships with international collaborators?
7. Project Summary/Abstract
8. Project Narrative
9. Bibliography & References Cited
10. Facilities & Other Resources
11. Equipment
12. Other Attachments
Additional Instructions for Fellowship:
This R&R Other Project Information Form should be completed in consultation with the sponsor and administrative officials at the sponsoring institution.
1. Are Human Subjects Involved?
This field is required.
If activities involving human subjects are planned at any time during the proposed project at any performance site, check "Yes." Check "Yes" even if the proposed project is exempt from regulations for the Protection of Human Subjects, or if activities involving human subjects are anticipated within the period of award but plans are indefinite, or if the proposed activities include public health surveillance activities described in 45 CFR 46.102(l)(2).
If activities involving human subjects are not planned at any time during the proposed project at any performance site, select "No" and skip the rest of the "Are Human Subjects Involved" section.
Whether you answer "Yes" or "No" to the "Are Human Subjects Involved?" question here, your answer will populate the relevant field in the G.500 - PHS Human Subjects and Clinical Trials Information form (see exception for Training Applications in the Training-specific instructions). Follow the G.500 - PHS Human Subjects and Clinical Trials Information form instructions to complete the relevant questions in that form.
Additional Instructions for Training:
K12 and D43 applicants: The general instructions above apply to you (i.e., your answer to "Are Human Subjects Involved?" will populate the relevant field in the PHS Human Subjects and Clinical Trials Information form).
All other Training applicants: The PHS Human Subjects and Clinical Trials Information form is not applicable and will not be available to you.
Need help determining whether your application includes human subjects? Check out the NIH Research Involving Human Subjects website for information, including an Infopath Questionnaire designed to walk applicants through the decision process.
Note on the use of human specimens or data: Applications involving the use of human specimens or data may or may not be considered to be research involving human subjects, depending on the details of the materials to be used. If you check "No" to "Are Human Subjects Involved?" but your application proposes using human specimens or data, you will be required to provide a clear justification about why this use does not constitute human subjects research. Follow the G.500 - PHS Human Subjects and Clinical Trials Information form instructions.
For more information on human biospecimens or data: Refer to the NIH page on Frequently Asked Questions on Human Specimens, Cell Lines, or Data and the Research Involving Private Information or Biological Specimens flowchart.
Additional Instructions for Training:
Check "Yes" if training plans include or potentially will include involvement of trainees in projects that include human subjects as defined by 45 CFR 46.
Most Training grant application packages do not include the G.500 - PHS Human Subjects and Clinical Trials Information form. Although it is not required, applicants can provide additional information regarding potential or current involvement of appointed trainees in human subjects research in the "Proposed Training" section of the Program Plan attachment on the G.420 - PHS Research Training Program Plan Form.
In many instances, trainees supported by institutional training grants will participate in research that is supported by separate research project grants for which Institutional Review Board (IRB) approval or a determination of exemption exists. Existing IRB approval may be sufficient for trainees, provided that the IRB determines the research would not be substantially modified by the participation of a trainee.
Trainees may participate only in non-exempt human subjects research that is being conducted by an institution that has an approved Federalwide Assurance (FWA) on file with the Office for Human Research Protections (OHRP) and that has IRB approval. The awardee institution is responsible for maintaining documentation of FWA and IRB approvals for all trainee research projects and for providing this information to NIH if requested.
Trainees may not design or conduct independent human subjects research as part of the training award unless the institution where the research will be conducted has an approved FWA on file with OHRP and IRB approval. The institution must also submit certification of the date of IRB approval and must comply with NIH requirements for human subjects protections (see the NIH Grants Policy Statement, Section 4.1.15: Human Subjects Protections).
Trainees who will be involved in the design or conduct of research involving human subjects must receive training in human subjects protections. It is the institution's responsibility to ensure that trainees are properly supervised when working with human subjects.
These policies apply to all Performance Sites.
K12 and D43 applicants applying to FOAs that accept clinical trials (e.g., 'clinical trial optional'): Follow the instructions in your FOA to determine whether you must provide information in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Additional Instructions for Fellowship:
In many instances, the fellow will be participating in research supported by a research project grant for which the IRB review of human subjects is already complete or an exemption has been designated. This review or exemption designation is sufficient, provided that the IRB determines that participation of the Fellow does not substantially modify the research.
Additional Instructions for Multi-project:
Overall Component: If activities involving human subjects are planned at any time during the proposed project at any performance site and/or on any Other Component, check "Yes" to the "Are Human Subjects Involved?" question and complete the remaining questions as instructed.
Other Components: Answer only the "Are Human Subjects Involved?" and "Is the Project Exempt from Federal regulations?" questions.
1.a. If YES to Human Subjects
Your answers here in question "1.a. If YES to Human Subjects" will populate the corresponding fields in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Is the Project Exempt from Federal regulations? Yes/No
If the project is exempt from federal regulations, check "Yes" and check the appropriate exemption number.
Human subjects research should only be designated as exempt if all of the proposed research projects in an application meet the criteria for exemption.
If the project is not exempt from federal regulations, check "No."
For more information, see the NIH's Exempt Human Subjects Research infographic.
If yes, check appropriate exemption number 1, 2, 3, 4, 5, 6, 7, 8:
If you selected "Yes" to "Is the Project Exempt from Federal Regulations," select the appropriate exemption number.
The categories of research that qualify for exemption are defined in the Common Rule for the Protection of Human Subjects. These regulations can be found at 45 CFR 46.
Need help determining the appropriate exemption number? Refer to NIH's Research Involving Human Subjects Frequently Asked Questions.
The Office for Human Research Protections (OHRP) guidance states that appropriate use of exemptions described in 45 CFR 46 should be determined by an authority independent from the investigators (for more information, see OHRP's Frequently Asked Questions). Institutions often designate their Institutional Review Board (IRB) to make this determination. Because NIH does not require IRB approval at the time of application, the exemptions designated often represent the opinion of the PD/PI, and the justification provided for the exemption by the PD/PI is evaluated during peer review. See NIH Grants Policy Statement Section 4.1.15 for more information.
Notes on public health surveillance activities: Projects involving public health surveillance activities described in 45 CFR 46.102(l)(2) must answer questions in Section 1.a. as if the exclusion does not apply. In rare circumstances, applicants may request NIH approval for use of the exclusion in accordance with Just-in-Time procedures.
Additional Instructions for Multi-project:
Overall Component: Check all the exemptions identified in all the Other Components.
Other Components: If the Overall Component exemption is only E4 (box 4 is checked) then no other exemption number can be set for any Other Component.
If no, is the IRB review Pending? Yes/No
If IRB review is pending, check "Yes."
Applicants should check "Yes" to the question "Is the IRB review Pending?" even if the IRB review/approval process has not started by the time of submission.
If IRB review is not pending (e.g., if the review is complete), check "No."
Additional Instructions for Multi-project:
Other Components: Skip the "If no, is the IRB review Pending?" question.
IRB Approval Date:
Enter the latest IRB approval date (if available). Leave blank if IRB approval is pending.
An IRB approval date is not required at the time of submission when IRB review is pending. This may be requested later in the pre-award cycle as a Just-In-Time requirement. See the NIH Grants Policy Statement, Section 2.5.1: Just-in-Time Procedures for more information.
Additional Instructions for Multi-project:
Other Components: Skip the "IRB Approval Date" question.
Human Subject Assurance Number:
Enter the approved Federalwide Assurance (FWA) number that the applicant has on file with OHRP. Enter the 8-digit number. Do not enter "FWA" before the number.
Enter "None" if the applicant organization does not have an approved FWA on file with OHRP. In this case, the applicant organization, by the signature in the Certification section on the G.200 - SF424 (R&R) Form, is declaring that it will comply with 45 CFR 46 and proceed to obtain a FWA (see Office for Human Research Protections website). Do not enter the FWA number of any collaborating institution.
Additional Instructions for Fellowship:
If research proposed in the fellowship application has been previously reviewed and approved by an IRB and is covered by an approved FWA, provide the FWA number and the latest IRB approval date for the proposed activities. The latest IRB approval date must be within one year of the application due date.
Additional Instructions for Multi-project:
Other Components: Skip the "Human Subject Assurance Number" field.
2. Are Vertebrate Animals Used?
This field is required.
If activities involving vertebrate animals are planned at any time during the proposed project at any performance site, check "Yes." Otherwise, check "No" and skip the rest of the "2. Are Vertebrate Animals Used?" section.
Note that the generation of custom antibodies constitutes an activity involving vertebrate animals.
If animal involvement is anticipated within the period of award but plans are indefinite, check "Yes."
Additional Instructions for Research:
If you have answered "Yes" to the "Are Vertebrate Animals Used?" question, you must also provide an explanation and anticipated timing of animal use in G.400 - PHS 398 Research Plan Form, Vertebrate Animals. This attachment must be submitted and reviewed prior to the involvement of animals in any research studies.
Additional Instructions for Career Development:
If you have answered "Yes" to the "Are Vertebrate Animals Used?" question, you must also provide an explanation and anticipated timing of animal use in G.410 - PHS 398 Career Development Award Supplemental Form, Vertebrate Animals. This attachment must be submitted and reviewed prior to the involvement of animals in any research studies.
Additional Instructions for Training:
In many instances, trainees supported by institutional training grants will participate in research that is supported by a separate research project grant for which the IACUC review and approval exist. This existing IACUC approval is sufficient for trainees provided that the research would not be substantially modified by the participation of a trainee.
Note that trainees may only participate in vertebrate animal research that is being conducted at an institution that has an approved Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare (OLAW) and that has IACUC approval. The awardee institution is responsible for maintaining documentation of the Animal Welfare Assurance and IACUC approvals for all trainee research projects and providing this information to NIH if requested.
Trainees may not design or conduct independent vertebrate animal research as part of the training award unless the institution has an approved Animal Welfare Assurance on file with OLAW and IACUC approval has been obtained. Verification of IACUC approval (within 3 years) must be submitted to NIH, and NIH requirements for research involving vertebrate animals must be addressed.
Prior to conducting any animal activities, the grantee must submit the detailed information about the use of animals as required in the instructions in G.420 - PHS 398 Research Training Program Plan, Vertebrate Animals. This detailed information must be submitted to the NIH awarding IC for prior approval.
The institution must ensure that trainees are enrolled in the institution's animal welfare training and occupational health and safety programs for personnel who have contact with animals. It is the institution's responsibility to ensure that trainees are properly supervised when working with live vertebrate animals.
These policies apply to all Performance Sites.
Additional Instructions for Fellowship:
In many instances, the fellow will be participating in research supported by a research project grant for which the IACUC review has been completed and approval obtained. This review is sufficient, provided that participation of the fellow does not substantially modify the research. The appropriate grant(s) must be identified along with the IACUC approval date(s).
The sponsoring institution must ensure that the fellow is enrolled in the institution's animal welfare training and safety programs for personnel who have contact with animals, as appropriate. It is also the sponsoring institution's responsibility to ensure that the fellow is properly supervised when working with live vertebrate animals.
If you have answered "Yes" to the "Are Vertebrate Animals Used?" question, you must also provide an explanation and anticipated timing of animal use in G.430 - PHS Fellowship Supplemental Form, Vertebrate Animals. This attachment must be submitted and reviewed prior to the involvement of animals in any research studies.
Additional Instructions for Multi-project:
Overall Component: If activities involving vertebrate animals are planned at any time during the proposed project at any performance site and/or on any Other Component, check "Yes" and complete the remaining questions as instructed.
Other Components: Answer only the "Are Vertebrate Animals Used?" question. Skip the questions in 2.a.
Additional Instructions for SBIR/STTR:
![]() The small business applicant needs to answer "Yes" even if vertebrate animal work will not be performed at the small business. If you have answered "Yes" to the "Are Vertebrate Animals Used?" question, you must also provide an explanation and anticipated timing of animal use in G.400 - PHS 398 Research Plan Form, Vertebrate Animals. This attachment must be submitted and reviewed prior to the involvement of animals in any research studies.
The small business applicant needs to answer "Yes" even if vertebrate animal work will not be performed at the small business. If you have answered "Yes" to the "Are Vertebrate Animals Used?" question, you must also provide an explanation and anticipated timing of animal use in G.400 - PHS 398 Research Plan Form, Vertebrate Animals. This attachment must be submitted and reviewed prior to the involvement of animals in any research studies.
2.a. If YES to Vertebrate Animals
Is the IACUC review Pending?
If an Institutional Animal Care and Use Committee (IACUC) review is pending, check "Yes."
Applicants should check "Yes" to the "Is the IACUC review Pending?" question even if the IACUC review/approval process has not started by the time of submission.
If IACUC review is not pending (e.g. if the review is complete), check "No."
Additional Instructions for Multi-project:
Overall Component: Complete the "Is the IACUC review Pending?" question when the answer is "Yes" to "Are Vertebrate Animals Used?"
Other Components: Skip the "Is the IACUC review Pending?" question.
IACUC Approval Date:
Enter the latest IACUC approval date (if available). Leave blank if IACUC approval is pending. IACUC approval must have been granted within three years of the application submission date to be valid.
An IACUC approval date is not required at the time of submission. NIH does not require verification of review and approval of the proposed research by the IACUC before peer review of the application. However, this information is required under the NIH Grants Policy Statement Section 2.5.1: Just-in-Time Procedures.
Additional Instructions for Multi-project:
Other Components: Skip the "IACUC Approval Date" question.
Animal Welfare Assurance Number
Enter the federally approved assurance number, if available.
Enter "None" if the applicant organization does not have an Office of Laboratory Animal Welfare (OLAW)-approved Animal Welfare Assurance.
To determine whether the applicant organization holds an Animal Welfare Assurance with an associated number, see the lists of Domestic and Foreign Assured institutions. Do not enter the Animal Welfare Assurance number for a Project/Performance Site of a collaborating institution.
When an applicant organization does not have an Animal Welfare Assurance number, the authorized organization representative's signature on the application constitutes declaration that the applicant organization will submit an Animal Welfare Assurance when requested by OLAW.
If the animal work will be conducted at an institution with an Animal Welfare Assurance and the applicant organization does not have the following:
- an animal care and use program;
- facilities to house animals and conduct research on site; and
- IACUC;
then, the applicant must obtain an Inter-institutional Assurance from OLAW prior to an award.
Additional Instructions for Multi-project:
Other Components: Skip the "Animal Welfare Assurance Number" question.
3. Is proprietary/privileged information included in the application?
This field is required.
Patentable ideas; trade secrets; or privileged, confidential commercial, or financial information should be included in applications only when such information is necessary to convey an understanding of the proposed project.
If the application includes such information, check "Yes" and clearly mark each line or paragraph on the pages containing the proprietary/privileged information with a statement similar to: "The following contains proprietary/privileged information that (name of applicant) requests not be released to persons outside the government, except for purposes of review and evaluation." This statement can be included at the top of each page as applicable.
If a grant is awarded as a result of or in connection with the submission of this application, the government shall have the right to use or disclose the information to the extent authorized by law. Although the grantee institution and the PD/PI will be consulted about any such disclosure, the NIH and other PHS agencies will make the final determination. Any indication by the applicant that the application contains proprietary or privileged information does not automatically shield the information from release in response to a Freedom of Information Act (FOIA) request should the application result in an award (see 45 CFR 5). Additionally, if an applicant fails to identify proprietary information at the time of submission as instructed here, a significant substantive justification will be required to withhold the information if requested under FOIA.
4. Environmental Questions
Question 4 pertains to the environmental impact of the proposed research.
4.a. Does this Project Have an Actual or Potential Impact - positive or negative - on the environment?
This field is required.
Indicate whether or not this project has an actual or potential impact on the environment.
Most NIH research grants are not expected to individually or cumulatively have a significant effect on the environment, and NIH has established several categorical exclusions allowing most applicants to answer "No" unless a specific FOA indicates that the National Environmental Policy Act (NEPA) applies. However, if an applicant expects that the proposed project will have an actual or potential impact on the environment, or if any part of the proposed research and/or project includes one or more of the following scenarios, check "Yes."
- The potential environmental impacts of the proposed research may be of greater scope or size than other actions included within a category.
- The proposed research threatens to violate a federal, state, or local law established for the protection of the environment or for public health and safety.
- Potential effects of the proposed research are unique or highly uncertain.
- Use of especially hazardous substances or processes is proposed for which adequate and accepted controls and safeguards are unknown or not available.
- The proposed research may overload existing waste treatment plants due to new loads (volume, chemicals, toxicity, additional hazardous wasted, etc.).
- The proposed research may have a possible impact on endangered or threatened species.
- The proposed research may introduce new sources of hazardous/toxic wastes or require storage of wastes pending new technology for safe disposal.
- The proposed research may introduce new sources of radiation or radioactive materials.
- Substantial and reasonable controversy exists about the environmental effects of the proposed research.
4.b. If yes, please explain:
If you answered "Yes" to Question 4.a., you must provide an explanation here as to the actual or potential impact of the proposed research on the environment. Your entry is limited to 55 characters.
4.c. If this project has an actual or potential impact on the environment, has an exemption been authorized or an environmental assessment (EA) or environmental impact statement (EIS) been performed? Yes/No.
This field is required if you answered "Yes" to Question 4.a. Check "Yes" or "No."
4.d. If yes, please explain:
Enter additional details about the EA or EIS here. Your entry is limited to 55 characters.
5. Is the research performance site designated, or eligible to be designated, as a historic place?
This field is required.
If any research performance site is designated, or eligible to be designated, as a historic place, check the "Yes" box. Otherwise, check "No."
5.a. If yes, please explain:
If you checked "Yes" to indicate that any performance site is designated, or eligible to be designated, as a historic place, provide the explanation here. Your entry is limited to 55 characters.
6. Does this project involve activities outside of the United States or partnerships with international collaborators?
This field is required.
Indicate whether this project involves activities outside of the United States or partnerships with international collaborators. Check "Yes" or "No."
Applicants to NIH and other PHS agencies must check "Yes" if the applicant organization is a foreign institution or if the project includes a foreign component. See NIH Glossary for a definition of a foreign component.
If you have checked "Yes" to Question 6, you must include a "Foreign Justification" attachment in Field 12, Other Attachments. Describe special resources or characteristics of the research project (e.g., human subjects, animals, disease, equipment, and techniques), including the reasons why the facilities or other aspects of the proposed project are more appropriate than a domestic setting. In the body of the text, begin the section with a heading indicating "Foreign Justification" and name the file "Foreign Justification."
Additional Instructions for Fellowship:
If you have checked "Yes" to Question 6, your justification must include a description of how the foreign training is more appropriate than in a domestic setting. Include reasons why the facilities, the sponsor, and/or other aspects of the proposed experience are more appropriate in a foreign setting. The justification is evaluated in terms of the scientific advantages of the foreign training as compared to the training available domestically. Foreign training will be considered for funding only when the scientific advantages are clear. The foreign justification should be provided as a separate attachment in the "12. Other Attachments" section in G.220 - R&R Other Project Information Form.
Additional Instructions for Multi-project:
Overall Component: If the answer to Question 6 is "Yes" for any Other Component, then you must answer "Yes" for the Overall Component.
Additional Instructions for SBIR/STTR:
In rare and unique circumstances, for example, if a supply or material or the study design (e.g., patient population) is not available in the United States, NIH may allow a small portion of the work to be performed by a foreign organization. Foreign involvement will be considered on a case-by-case basis and must be thoroughly justified in the application. Applicants should discuss their unique situations with their NIH program official prior to submission of the application. Whenever possible, non-SBIR/STTR funds should be used for other work outside of the United States that is necessary to the overall completion of the project.
6.a. If yes, identify countries:
This field is required if you answered "Yes" to Question 6. Enter the countries with which international cooperative activities are planned.
You may use abbreviations. Your entry is limited to 55 characters.
6.b. Optional Explanation:
This field is optional. Enter an explanation for involvement with outside entities. Your entry is limited to 55 characters.
7. Project Summary/Abstract
The "Project Summary/Abstract" attachment is required.
The project summary is a succinct and accurate description of the proposed work and should be able to stand on its own (separate from the application). This section should be informative to other persons working in the same or related fields and understandable to a scientifically literate reader. Avoid both descriptions of past accomplishments and the use of the first person. Please be concise.
Format:
This section is limited to 30 lines of text, and must follow the required font and margin specifications. A summary that exceeds the 30-line limit will be flagged as an error by the Agency upon submission. Use of hyperlinks and URLs in this section is not allowed unless specified in the funding opportunity announcement.
Attach this information as a PDF file. See the Format Attachments page.
Content:
State the application's broad, long-term objectives and specific aims, making reference to the health relatedness of the project (i.e., relevance to the mission of the agency). Describe the research design and methods for achieving the stated goals. Be sure that the project summary reflects the key focus of the proposed project so that the application can be appropriately categorized.
Do not include proprietary, confidential information or trade secrets in the project summary. If the application is funded, the project summary will be entered into an NIH database and made available on the NIH Research Portfolio Online Reporting Tool (RePORT) and will become public information.
Note that the "Project Summary/Abstract" attachment is not same as the "Research Strategy" attachment.
Additional Instructions for Career Development:
In addition to summarizing the research project to be conducted under the career development award, describe the candidate's career development plan, the candidate's career goals, and the environment in which the career development will take place. The entire "Project Summary/Abstract" attachment is limited to 30 lines of text.
Additional Instructions for Training:
In addition to the content described above, also summarize the objectives, rationale and design of the research training program. Provide information regarding the research areas and scientific disciplines encompassed by the program. Include a brief description of the level(s) (i.e., undergraduate, predoctoral, postdoctoral, faculty) and duration of the proposed training, and the projected number of participating trainees. The entire "Project Summary/Abstract" attachment is limited to 30 lines of text.
Additional Instructions for Fellowship:
In addition to summarizing the research project to be conducted under the fellowship award, describe the fellowship training plan and the environment in which the research training will take place. The entire "Project Summary/Abstract" attachment is limited to 30 lines of text.
Additional Instructions for Multi-project:
Overall and Other Components: A project summary is required for both the Overall Component and all Other Components. Each project summary attachment is limited to 30 lines of text.
8. Project Narrative
The "Project Narrative" attachment is required.
Content:
Describe the relevance of this research to public health in, at most, three sentences. For example, NIH applicants can describe how, in the short or long term, the research would contribute to fundamental knowledge about the nature and behavior of living systems and / or the application of that knowledge to enhance health, lengthen life, and reduce illness and disability. Use of hyperlinks and URLs in this section is not allowed unless specified in the funding opportunity announcement. If the application is funded, this public health relevance statement will be combined with the project summary (above) and will become public information.
Additional Instructions for Multi-project:
Overall Component: The "Project Narrative" attachment is required.
Other Components: Refer to the specific FOA to determine whether the "Project Narrative" attachment is required for any Other Components. Note: The form may show ‘*' indicating it is a required field, but it is only required for the Overall Component and the ‘*' can be ignored for Other Components.
9. Bibliography & References Cited
Who must complete the "Bibliography & References Cited" attachment:
The "Bibliography & References Cited" attachment is required unless otherwise noted in the FOA.
Format:
Attach this information as a PDF file. See the Format Attachments page. Use of hyperlinks and URLs in this section is not allowed unless specified in the funding opportunity announcement.
Content:
See the following instructions for which references to include in the "Bibliography and References Cited" attachment.
Additional Instructions for Research:
The "Bibliography & References Cited" attachment should include any references cited in G.400 - PHS 398 Research Plan Form and in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Additional Instructions for Career Development:
The "Bibliography & References Cited" attachment should include any references cited in G.410 - PHS 398 Career Development Award Supplemental Form and in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Additional Instructions for Training:
The "Bibliography & References Cited" Attachment should include any references cited in G.420 - PHS 398 Research Training Program Plan Form and in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Additional Instructions for Fellowship:
The "Bibliography & References Cited" attachment should include any references cited in G.430 - PHS Fellowship Supplemental Form and in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Additional Instructions for Multi-project:
Overall and Other Components: The "Bibliography & References Cited" attachment should include any references cited in G.400 - PHS 398 Research Plan Form and in the G.500 - PHS Human Subjects and Clinical Trials Information form.
Additional Instructions for SBIR/STTR:
The "Bibliography & References Cited" attachment should include any references cited in G.400 - PHS 398 Research Plan Form and in the G.500 - PHS Human Subjects and Clinical Trials Information form.
When citing articles that fall under the Public Access Policy, were authored or co-authored by the applicant, and arose from NIH support, provide the NIH Manuscript Submission reference number (e.g., NIHMS97531) or the PubMed Central (PMC) reference number (e.g., PMCID234567) for each article. If the PMCID is not yet available because the Journal submits articles directly to PMC on behalf of their authors, indicate "PMC Journal - In Process." NIH maintains a list of such journals.
Citations that are not covered by the Public Access Policy, but are publicly available in a free, online format may include URLs or PubMed ID (PMID) numbers along with the full reference. Active hyperlinks in this section are not allowed. The references should be limited to relevant and current literature. While there is not a page limitation, it is important to be concise and to select only those literature references pertinent to the proposed research.
You are allowed to cite interim research products. Note: interim research products have specific citation requirements. See related Frequently Asked Questions for more information.
Additional Instructions for Training:
The "Bibliography & References Cited" attachment should be used only to cite references supporting the need, rationale, and approach for the training program described in the G.420 - PHS 398 Research Training Program Plan. Do not include lists of publications of project directors, mentors or trainees in this section, as this information will be included in the Biosketches and Data Tables.
Additional Instructions for Multi-project:
Overall and Other Components: Unless specific instructions are provided in the FOA, applicants have the option of including the "Bibliography & References Cited" attachment in the Overall Component, Other Components, or both. User-defined bookmarks provided in the Bibliography & References Cited attachment will be included with the bookmarks of the assembled application image in eRA Commons. If you include the "Bibliography & References Cited" attachment only in the Overall Component, you may want to use bookmarks to organize references by component.
10. Facilities & Other Resources
Format:
The "Facilities & Other Resources" attachment is required unless otherwise specified in the FOA. Use of URLs and hyperlinks in this section is not allowed unless specified in the funding opportunity announcement.
Content:
Describe how the scientific environment in which the research will be done contributes to the probability of success (e.g., institutional support, physical resources, and intellectual rapport). In describing the scientific environment in which the work will be done, discuss ways in which the proposed studies will benefit from unique features of the scientific environment or from unique subject populations or how studies will employ useful collaborative arrangements.
If there are multiple performance sites, describe the resources available at each site.
Describe any special facilities used for working with biohazards and any other potentially dangerous substances. Note: Information about select agents must be described in the Research Plan, Select Agent Research.
For early stage investigators (ESIs), describe institutional investment in the success of the investigator. See NIH's New and Early Stage Investigator Policies. Your description may include the following elements:
- resources for classes, travel, or training;
- collegial support, such as career enrichment programs, assistance and guidance in the supervision of trainees involved with the ESI's project, and availability of organized peer groups;
- logistical support, such as administrative management and oversight and best practices training;
- financial support, such as protected time for research with salary support.
Additional Instructions for Career Development:
Include a detailed description of the institutional facilities and resources available to the candidate. The information provided is of major importance in establishing the feasibility of the goals of the career development plan.
Additional Instructions for Training:
Describe the facilities and resources that will be used in the proposed training program, including any foreign performance sites. Indicate how the applicant organization will support the program, financial or otherwise. This could include, for example, supplementation of stipends, shared space and/or laboratory facilities and equipment, funds for curriculum development, support for student activities, release time for the PD/PI and participating faculty (e.g., protected time for mentoring), support for additional trainees in the program, or any other creative ways to improve the environment for the establishment and growth of the research training program.
Additional Instructions for Fellowship:
Include a detailed description of the institutional facilities and resources available to the fellowship applicant. The information provided is of major importance in establishing the feasibility of the goals of the fellowship training plan.
Additional Instructions for Multi-project:
Unless specific instructions are provided in the FOA, applicants have the option of including the "Facilities & Other Resources" attachment in the Overall Component, Other Components, or both.
Additional Instructions for SBIR/STTR:
![]() The research to be performed by the applicant small business and its collaborators must be in United States facilities that are available to and under the control of each party for the conduct of each party's portion of the proposed project. Foreign sites must be approved by the awarding agency. In rare and unique circumstances, for example, if a supply or material or the study design (e.g., patient population) is not available in the United States, NIH may allow a small portion of the research or R&D work to be performed by a foreign organization. Foreign involvement will be considered on a case-by-case basis and must be thoroughly justified in the application. Applicants should discuss their unique situations with NIH program staff prior to submission of the application. Whenever possible, non-SBIR/STTR funds should be used for other work outside of the United States that is necessary to the overall completion of the project. Be sure to discuss any plans for inclusion of foreign institutions or sites with a Program Official.
The research to be performed by the applicant small business and its collaborators must be in United States facilities that are available to and under the control of each party for the conduct of each party's portion of the proposed project. Foreign sites must be approved by the awarding agency. In rare and unique circumstances, for example, if a supply or material or the study design (e.g., patient population) is not available in the United States, NIH may allow a small portion of the research or R&D work to be performed by a foreign organization. Foreign involvement will be considered on a case-by-case basis and must be thoroughly justified in the application. Applicants should discuss their unique situations with NIH program staff prior to submission of the application. Whenever possible, non-SBIR/STTR funds should be used for other work outside of the United States that is necessary to the overall completion of the project. Be sure to discuss any plans for inclusion of foreign institutions or sites with a Program Official.
11. Equipment
The "Equipment" attachment is required.
Format:
Attach this information as a PDF file. Use of URLs and hyperlinks in this section is not allowed unless specified by the funding opportunity announcement.
Content:
List major items of equipment already available for this project and, if appropriate, identify the equipment's location and pertinent capabilities.
Additional Instructions for Multi-project:
Unless specific instructions are provided in the FOA, applicants have the option of including the "Equipment" attachment in the Overall Component, Other Components, or both (whichever is most appropriate for your application). User-defined bookmarks provided in the Equipment attachment will be included with the bookmarks of the assembled application image in eRA Commons. If you include the "Equipment" attachment only in the Overall Component, you may want to use bookmarks to organize equipment by component.
12. Other Attachments
Attach a file to provide additional information only in accordance with the FOA and/or agency-specific instructions.
If applicable, attach a "Foreign Justification" here. (See Question 6 above).
Additional Instructions for SBIR/STTR:
NIH, CDC, SBIR, and CRP Applicants Only:
SBIR Application Certification for small business concerns that are majority-owned by multiple venture capital operating companies, hedge funds, or private equity firms (e.g. majority VCOC-owned): You are required to submit a VCOC Certification with your application per the SBIR Policy Directive.
Certain small business applicants do not have to fill out the VCOC Certification. Applicant small businesses that are more than 50% directly owned and controlled by one or more individuals (who are citizens or permanent resident aliens of the United States), other business concerns (each of which is more than 50% directly owned and controlled by individuals who are citizens or permanent resident aliens of the United States), or any combination of these (i.e. NOT majority VCOC-owned) should NOT fill out this certification and should NOT attach it to their application package.
- Download the "SBIR Application VCOC Certification" at the NIH Forms & Applications page.
- Answer the 3 questions and check the certification boxes.
- The authorized business official must sign the certification.
- Save the certification using the original filename ("SBIR Application VCOC Certification"). DO NOT CHANGE OR ALTER THE FILENAME OR TYPE. Changing the filename may cause delays in the processing of your application.
-
![Attach this VCOC Certification PDF with the file name 'SBIR Application VCOC Certification.pdf' in 12, Other Attachment Special Instructions for Foreign Organizations (Non-domestic [non-U.S.] Entities)](resources/images/doc_icon.png) Attach this VCOC Certification PDF with the file name "SBIR Application VCOC Certification.pdf" in 12, Other Attachments.
Attach this VCOC Certification PDF with the file name "SBIR Application VCOC Certification.pdf" in 12, Other Attachments.
