G.210 - PHS 398 Cover Page Supplement Form
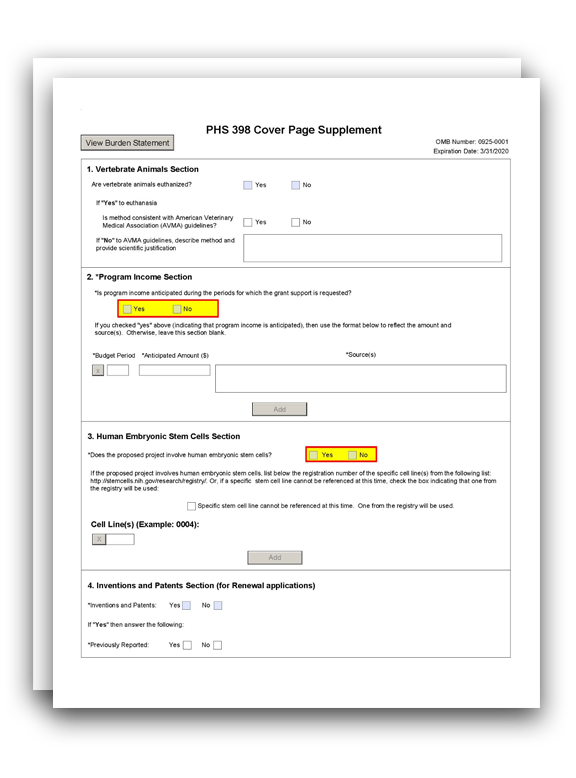
The PHS 398 Cover Page Supplement Form is used for all grant applications except fellowships. This form collects information on human subjects, vertebrate animals, program income, human embryonic stem cells, inventions and patents, and changes of investigator/change of institution.
2. Program Income Section
3. Human Embryonic Stem Cell Section
4. Human Fetal Tissue Section.
5. Inventions and Patents Section (for Renewal applications)
6. Change of Investigator/Change of Institution Section
1. Vertebrate Animals Section
Are vertebrate animals euthanized?
You must answer this question if you answered "Yes" to the question "Are Vertebrate Animals Used?" on the G.220 - R&R Other Project Information Form.
Check "Yes" or "No" to indicate whether vertebrate animals in the project are euthanized.
Additional Instructions for Multi-project:
Overall Component: If vertebrate animals will be euthanized in any Component, then you must answer "Yes" to the "Are vertebrate animals euthanized?" question.
If "Yes" to euthanasia: Is method consistent with American Veterinary Medical Association (AVMA) guidelines?
You must answer this question if you answered "Yes" to the "Are vertebrate animals euthanized?" question above. Check "Yes" or "No" to indicate whether the method of euthanasia is consistent with the AVMA Guidelines for the Euthanasia of Animals.
For more information: See AVMA Guidelines for the Euthanasia of Animals.
If "No" to AVMA guidelines, describe method and provide scientific justification:
If you answered "No" to the "Is method consistent with AVMA guidelines?" question, you must describe (in 1000 characters or fewer) the method of euthanasia and provide a scientific justification for its use. This justification will be reviewed by Office of Laboratory Animal Welfare (OLAW).
If you answered "Yes" to the "Is method consistent with AVMA guidelines" question, skip this question.
2. Program Income Section
Is program income anticipated during the periods for which the grant support is requested?
This field is required.
If program income is anticipated during the periods for which grant support is requested, check "Yes," and complete the rest of the "Program Income" section.
If no program income is anticipated, check "No" and skip the rest of the "Program Income" section.
Additional Instructions for Training:
Check "No" for the "Is program income anticipated during the periods for which the grant support is requested?" question.
Additional Instructions for Multi-project:
Overall Component: If you anticipate program income on any component, then answer "Yes." Skip the other fields, as any information provided in them will be discarded. Instead of program income information being provided in the Overall Component, a system-generated summary of all program income information that you provide in Other Components will be included in the summaries section of the assembled application image.
Other Component: If you anticipate program income on any component, then answer "Yes." Provide the budget period, anticipated amount, and source information.
Budget Period:
Enter the budget periods for which program income is anticipated. If the application is funded, the Notice of Grant Award will provide specific instructions regarding the use of such income.
Anticipated Amount ($):
Enter the amount of anticipated program income for each budget period listed.
Source(s):
Enter the source of anticipated program income for each budget period listed.
3. Human Embryonic Stem Cells Section
Use the following instructions to complete the fields in this section.
For additional guidance, see the NIH Grants Policy Statement, Section 4.1.13: Human Stem Cell Research.
Does the proposed project involve human embryonic stem cells?
This field is required.
If the proposed project involves human embryonic stem cells (hESC), check "Yes" and complete the rest of the "Human Embryonic Stem Cells" section.
- Use of the cell lines must be in accordance with the NIH Guidelines for Human Stem Cell Research.
If the proposed project does not involve hESC, check "No" and skip the rest of the "Human Embryonic Stem Cells" section.
Additional Instructions for Training:
Check "Yes" if training plans include or potentially will include involvement of trainees in projects that include hESC. Note that trainees may only conduct research with hESC lines that are approved for use in NIH-funded research; these cell lines are listed on the NIH hESC Registry. Use of the cell lines must be in accordance with the NIH Guidelines for Human Stem Cell Research.
Additional Instructions for Multi-project:
Overall Component: If human embryonic stem cells are used in any Component, then you must answer "Yes."
Specific stem cell line cannot be referenced at this time. One from the registry will be used.
If you will use hESC but a specific line from the NIH hESC Registry cannot be chosen at the time of application submission, check this box.
If you cannot specify which cell lines will be used at the time of application submission, specific cell line information will be required as Just-in-Time information prior to award.
Additional Instructions for Research:
If you cannot choose an appropriate cell line from the registry at this time, provide a justification in the G.400 - PHS 398 Research Plan Form, Research Strategy attachment.
Additional Instructions for Career Development:
If you cannot choose an appropriate cell line from the registry at this time, provide a justification in the G.410 - PHS 398 Career Development Award Supplemental Form, Research Strategy attachment.
Additional Instructions for Training:
When individual project hESC line information is requested as Just-in-time (JIT), the NIH will require information regarding project title, mentor, and specific cell line(s) from the registry (NIH hESC Registry) for each trainee utilizing human embryonic stem cells. Trainees may not participate in hESC related research until this information has been provided.
Additional Instructions for Multi-project:
Overall and Other Components: If you cannot choose an appropriate cell line from the registry at this time, provide a justification in the G.400 - PHS 398 Research Plan Form, Research Strategy attachment.
Additional Instructions for SBIR/STTR:
If you cannot choose an appropriate cell line from the registry at this time, provide a justification in the G.400 - PHS 398 Research Plan Form, Research Strategy attachment.
Cell Line(s):
List the 4-digit registration number of the specific cell line(s) from the NIH hESC Registry (e.g. 0123). Up to 200 lines can be added.
Additional Instructions for Multi-project:
Overall Component: Skip the "Cell Line(s)" field, as any information provided here will be discarded. Instead of cell line information being provided in the Overall Component, a system-generated summary of all cell line information that you provide in Other Components will be included in the summaries section of the assembled application image.
Other Component: Provide any cell line information relevant to the work being done in that component.
For more information:
See NIH's Stem Cell Information page for additional information on stem cells, Federal policy statements, and guidelines on federally funded stem cell research.
4. Human Fetal Tissue Section
Does the proposed project involve human fetal tissue from elective abortions?
This field is required.
If the proposed project involves the use of human fetal tissue obtained from elective abortions (HFT), check "Yes" and complete the rest of the "Human Fetal Tissue" section.
If the proposed project does not involve the use of human fetal tissue obtained from elective abortions (HFT), check "No" and skip the rest of the "Human Fetal Tissue" section.
Additional Instructions for Multi-project:
Overall Component: If the use of human fetal tissue obtained from elective abortions (HFT) are proposed in any Component, then you must answer "Yes."
If the answer is "yes" then provide the HFT Compliance Assurance:
If the proposed project involves the use of human fetal tissue obtained from elective abortions (HFT), the applicant must provide a letter, signed by the PD/PI, assuring the HFT donating organization or clinic adheres to the requirements of the informed consent process and documenting that HFT was not obtained or acquired for valuable consideration. The PDF-formatted letter must be named ‘HFTComplianceAssurance.pdf'.
If the answer is "yes" then provide the HFT Sample IRB Consent Form
If the proposed project involves the use of human fetal tissue obtained from elective abortions (HFT), provide a blank sample of the IRB-approved consent form. The PDF-formatted form must be a blank sample and named ‘HFTSampleIRBConsentForm.pdf'.
o The informed consent for use of HFT from elective abortions requires language that acknowledges informed consent for donation of HFT was obtained by someone other than the person who obtained the informed consent for abortion, that informed consent for donation of HFT occurred after the informed consent for abortion was obtained will not affect the method of abortion, and that no enticements, benefits, or financial incentives were used at any level of the process to incentivize abortion or the donation of HFT. The form must be signed by both the woman and the person who obtains the informed consent.
For further information on HFT policy refer to the NIH Grants Policy Statement, Section 2.3.7.11 Human Fetal Tissue from Elective Abortions, Section 4.1.14 Human Fetal Tissue Research and Section 4.1.14.2 Human Fetal Tissue from Elective Abortions.
5. Inventions and Patents Section (for Renewal applications)
Who must complete the "Invention and Patents" section:
Complete the "Inventions and Patents" section only if you are submitting a renewal application or a resubmission of a renewal application.
Inventions and Patents:
If no inventions were conceived or reduced to practice during the course of work under this project, check "No" and skip the remainder of the "Inventions and Patents" section.
If any inventions were conceived or first actually reduced to practice during the previous period of support, check "Yes."
NIH recipient organizations must promptly report inventions to the Division of Extramural Inventions and Technology Resources (DEITR) Branch of the Office of Policy for Extramural Research Administration (OPERA), OER, NIH, 6705 Rockledge Drive, Bethesda, MD 20892-2750, (301) 435-1986. You must report inventions in compliance with regulations at 37 CFR 401.14, which are described at Interagency Edison (iEdison). The grantee is required to submit reports electronically using iEdison. See the NIH Grants Policy Statement, Section 8.4.1.6: Invention Reporting.
Additional Instructions for Career Development:
Skip the "Inventions and Patents" section, as it is not applicable.
Additional Instructions for Training:
Skip the "Inventions and Patents" section, as it is not applicable.
Previously Reported:
If you answered "Yes" to the "Inventions and Patents" question, indicate whether this information has been reported previously to the NIH or PHS agency or to the applicant organization official responsible for patent matters.
6. Change of Investigator/Change of Institution Section
Change of Project Director/Principal Investigator:
Check this box if your application reflects a change in project director/principal investigator (PD/PI) from that indicated on your previous application or award. Note that this box not applicable to a new application, nor is a change in PD/PI permitted for revision applications.
For a multiple PD/PI application, check this box if this application represents a change in the contact PI.
If you check the box, fill in the rest of the "Change of PD/PI" section with the information for the former PD/PI according to the instructions below.
Additional Instructions for Career Development:
Skip the "Change of Project Director/Principal Investigator" section, as changes in PD/PI are not allowed for career development awards.
Additional Instructions for Fellowship:
Skip the "Change of Project Director/Principal Investigator" section, as changes in PD/PI are not allowed for fellowship awards.
Prefix:
Enter or select the prefix, if applicable, for the former PD/PI.
First Name:
Enter the first (given) name of the former PD/PI.
Middle Name:
Enter the middle name of the former PD/PI.
Last Name:
Enter the last (family) name of the former PD/PI.
Suffix:
Enter or select the suffix, if applicable, for the former PD/PI.
Change of Grantee Institution:
Check this box if your application reflects a change in grantee institution from that indicated on your previous application or award. This question is not applicable to new applications.
Name of Former Institution:
Enter the name of the former institution if this application reflects a change in grantee institution.
