Revised: December 7, 2018
G.200 - SF 424 (R&R) Form
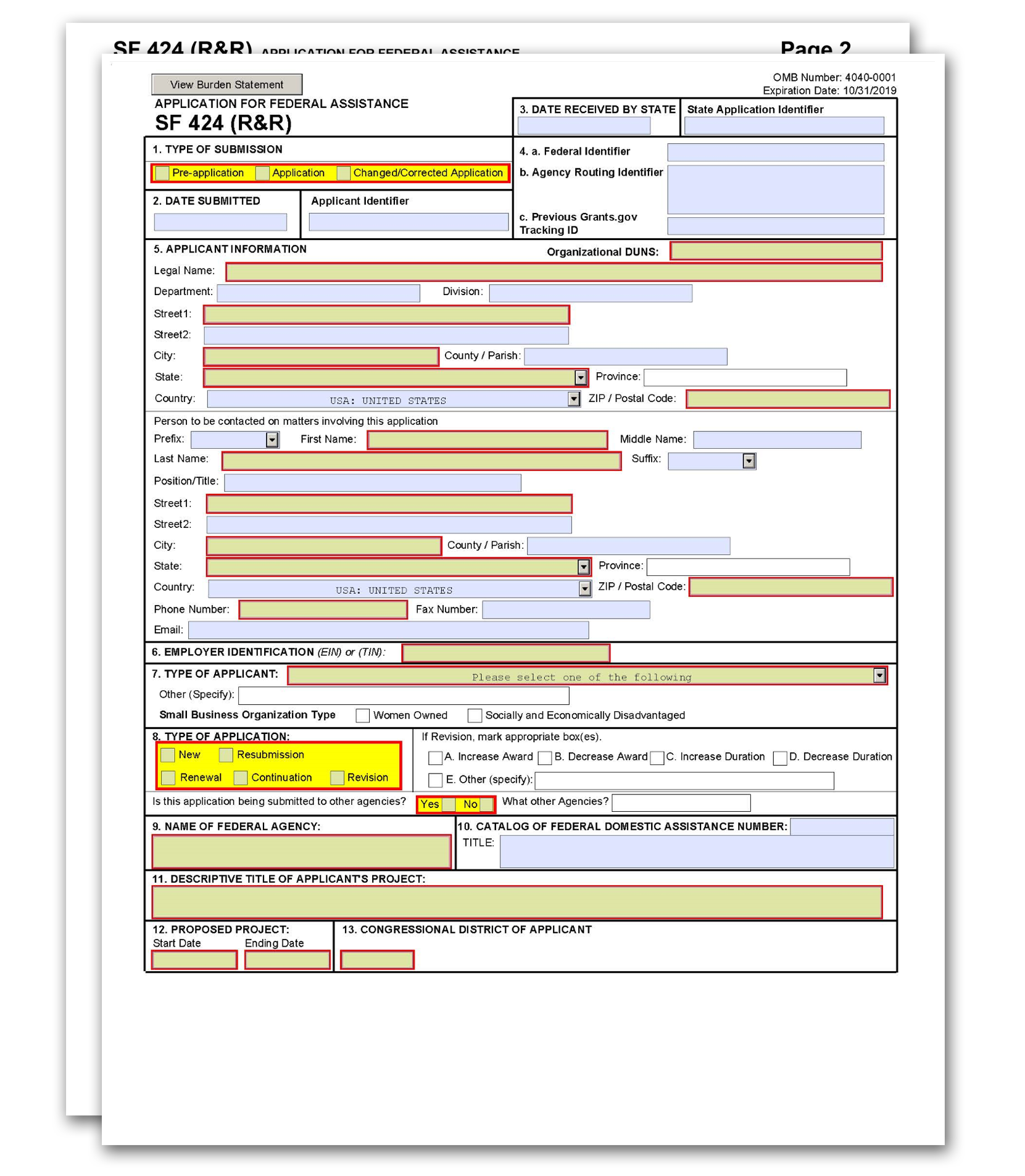
The SF 424 (R&R) Form is used in all grant applications. This form collects information including type of submission, applicant information, type of applicant, and proposed project dates.
2. Date Submitted and Applicant Identifier
3. Date Received by State and State Application Identifier
4a. Federal Identifier
4b. Agency Routing Identifier
4c. Previous Grants.gov Tracking ID
5. Applicant Information
6. Employer Identification
7. Type of Applicant
8. Type of Application
9. Name of Federal Agency
10. Catalog of Federal Domestic Assistance Number and Title
11. Descriptive Title of Applicant's Project
12. Proposed Project
13. Congressional District of Applicant
14. Project Director/Principal Investigator Contact Information
15. Estimated Project Funding
16. Is Application Subject to Review by State Executive Order 12372 Process?
17. Certification
18. SFLLL (Disclosure of Lobbying Activities) or Other Explanatory Documentation
19. Authorized Representative
20. Pre-application
21. Cover Letter Attachment
Additional Instructions for Multi-project:
Overall Component: Fill in all the SF424 (R&R) Form fields, as they are all collected.
Other Components: You need to fill in only a subset of fields in the SF424 (R&R) Form. Skip the other fields, as any information provided in them will be discarded. The fields you must fill in are:
- 5. Applicant Information
- 7. Type of Applicant (Optional)
- 11. Descriptive Title of Applicant's Project
- 12. Proposed Project
1. Type of Submission
This field is required. Check one of the "Type of Submission" boxes:
Pre-application:
The pre-application option is not used by NIH or other PHS agencies unless specifically noted in a funding opportunity announcement (FOA).
Application:
An "Application" is a request for financial support of a project or activity submitted on specified forms and in accordance with NIH instructions. (See NIH Types of Applications for an explanation of the types of applications).
Changed/Corrected Application:
Check this box if you are correcting either system validation errors or application assembly problems that occurred during the submission process. Changed/corrected applications must be submitted before the application due date.
When you submit a changed/corrected application, follow these guidelines:
- After submission of an application, there is a two-day application viewing window. Prior to the due date, you may submit a changed/corrected application. Submitting a changed/corrected application will replace the previous submission and remove the previous submission from consideration.
- If you check the "Changed/Corrected Application" box, then "Field 4.c Previous Grants.gov Tracking ID" is required.
- Do not use the "Changed/Corrected Application" box to denote a resubmission application. Resubmission applications will be indicated in "Field 8. Type of Application." See NIH Glossary for the definition of Resubmission.
Additional Instructions for SBIR/STTR:
SBIR/STTR Phase II/IIB Applications: To maintain eligibility to seek Phase II or IIB support, a Phase I awardee should submit a Phase II application, and a Phase II awardee should submit a Phase IIB application, within the first six due dates following the expiration of the Phase I or II budget period, respectively.
2. Date Submitted and Applicant Identifier
The "Date Submitted" field will auto-populate upon application submission.
Fill in the "Applicant Identifier" field, if applicable. The Applicant Identifier is reserved for applicant use, not the federal agency to which the application is being submitted.
3. Date Received by State and State Application Identifier
Skip the "Date Received by State" and "State Application Identifier" fields.
4.a. Federal Identifier
New Applications without Pre-application: Leave this field blank.
New Applications following Pre-application: Enter the agency-assigned pre-application number.
Resubmission, Renewal, and Revision Applications: The Federal Identifier is required. Include only the IC and serial number of the previously assigned application/award number (e.g., use CA987654 from 1R01CA987654-01A1).
Additional Instructions for SBIR/STTR:
When submitting a Phase II application, enter the Phase I SBIR/STTR grant number in this field.
For more information on applying for SBIR/STTR Phase II or Phase IIB awards, see SBIR/STTR Frequently Asked Questions.
4.b. Agency Routing Identifier
Skip the "Agency Routing Identifier" field unless otherwise specified in the FOA.
4.c. Previous Grants.gov Tracking ID
The "Previous Grants.gov Tracking ID" field is required if you checked the "Changed/Corrected Application" box in "Field 1. Type of Submission." A Tracking ID number is of the form, for example, GRANT12345678.
5. Applicant Information
The "Applicant Information" fields reflect information for the applicant organization, not a specific individual.
Additional Instructions for Multi-project:
Other Components: The "Applicant Information" section is required and applies to the lead organization of the component.
Additional Instructions for SBIR/STTR:
The small business concern is always the applicant organization for an SBIR or STTR award (e.g., ABC Incorporated).
The small business concern must be located in the United States.
Organizational DUNS:
This field is required.
Enter the DUNS or DUNS+4 number of the applicant organization.
This DUNS or DUNS+4 number must match the number entered in the eRA Commons Institutional Profile (IPF) for the applicant organization. The applicant's Authorized Organization Representative (AOR) is encouraged to confirm that a DUNS has been entered into the eRA Commons IPF prior to application submission. The same DUNS should be used in the eRA Commons IPF, Grants.gov, System for Award Management (SAM) registration, and in the DUNS field in the application.
If your organization does not already have a DUNS number, you will need to go to the Dun & Bradstreet website to obtain the number.
Additional Instructions for Multi-project:
Other Components: If a component is led by an organization other than the applicant organization, then you must provide the lead organization's DUNS or DUNS+4. However, the lead organization does not need to be registered in SAM or in eRA Commons at the time of application. SAM registration is encouraged since it helps staff process your application if you are selected for funding.
Legal Name:
Enter the legal name of the organization.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level within the organization.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level within the organization.
Street1:
This field is required. Enter the first line of the street address for the applicant organization.
Street2:
Enter the second line of the street address for the applicant organization.
City:
This field is required. Enter the city for the address of the applicant organization.
County/Parish:
Enter the county/parish for the address of the applicant organization.
State:
This field is required if the applicant organization is located in the United States or its territories. Enter the state or territory where the applicant organization is located.
Province:
If "Country" is Canada, enter the province of the applicant organization; otherwise, skip the "Province" field.
Country:
This field is required. Select the country for the address of the applicant organization.
ZIP/Postal Code:
The ZIP+4 is required if the applicant organization is located in the United States. Otherwise, the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the applicant organization.
Person to be contacted on matters involving this application
This information is for the administrative contact (e.g., AOR or business official), not the PD/PI. This person is the individual to be notified if additional information is needed and/or if an award is made.
Prefix:
Enter or select the prefix, if applicable, for the name of the person to contact on matters related to this application.
First Name:
This field is required. Enter the first (given) name of the person to contact on matters related to this application.
Middle Name:
Enter the middle name of the person to contact on matters related to this application.
Last Name:
This field is required. Enter the last (family) name of the person to contact on matters related to this application.
Suffix:
Enter or select the suffix, if applicable, for the name of the person to contact on matters related to this application.
Position/Title:
Enter the position/title for the person to contact on matters related to this application.
Street1:
This field is required. Enter the first line of the street address for the person to contact on matters related to this application.
Street2:
Enter the second line of the street address for the person to contact on matters related to this application.
City:
This field is required. Enter the city for the address of the person to contact on matters related to this application.
County/Parish:
Enter the county/parish for the address of the person to contact on matters related to this application.
State:
This field is required if the person to contact on matters related to this application is located in the United States or its Territories. Enter the state or territory where the person to contact on matters related to this application is located.
Province:
If "Country" is Canada, enter the province for the person to contact on matters related to this application; otherwise, skip the "Province" field.
Country:
Select the country for the address of the person to contact on matters related to this application.
ZIP/Postal Code:
The ZIP+4 is required if the person to contact on matters related to this application is in the United States. Otherwise, the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the person to contact on matters related to this application.
Phone Number:
This field is required. Enter the daytime phone number for the person to contact on matters related to this application.
Fax Number:
Enter the fax number for the person to contact on matters related to this application.
E-mail:
Enter the e-mail address for the person to contact on matters related to this application. Only one e-mail address is allowed, but it may be a distribution list.
6. Employer Identification
This field is required.
Enter either the organization's Taxpayer Identification Number (TIN) or Employer Identification Number (EIN) as assigned by the Internal Revenue Service. If your organization is not in the United States, enter 44-4444444. Your EIN may be 12 digits, and if this is the case, enter all 12 digits.
Additional Instructions for SBIR/STTR:
The small business must be located in the United States or a U.S. territory.
7. Type of Applicant
This field is required.
In the first field under "7. Type of Applicant," enter the appropriate applicant type. If your applicant type is not specified (e.g., for eligible Agencies of the Federal Government), select "X: Other (specify)," and indicate the name (e.g., the appropriate federal agency) in the space below.
Additional Instructions for Fellowship:
The information in "7. Type of Applicant" is for the applicant organization, not a specific individual authorized organization representative (AOR) or fellowship PD/PI.
Additional Instructions for Multi-project:
Other Components: You may fill out "7. Type of Applicant," but it is optional.
Additional Instructions for SBIR/STTR:
Select "R. Small Business." Also note whether the organization is Woman-owned and/or Socially and Economically Disadvantaged.
The applicant organization must certify (through Just-in-Time pre-award procedures) that it will qualify as a small business concern at the time of award.
Other (Specify):
Complete only if "X. Other (specify)" is selected as the "Type of Applicant."
Women Owned:
Check this box only if both "Small Business" is selected as the "Type of Applicant" and it is applicable. Woman-owned small businesses are small businesses that are at least 51% owned by a woman or women, who also control and operate it.
Socially and Economically Disadvantaged:
Check this box only if both "Small Business" is selected as the "Type of Applicant" and it is applicable. Socially and economically disadvantaged status is determined by the U.S. Small Business Administration pursuant to Section 8(a) of the Small Business Act U.S.C. 637(a).
8. Type of Application
This field is required.
Select the type of application. Check only one application type. Use the following list of existing definitions to determine what application type you have. For more information, see NIH Types of Applications.
- New. Check this option when submitting an application for the first time or in accordance with other submission policies. See the NIH Grants Policy Statement, Section 2.3.7.4: Submission of Resubmission Application.
- Resubmission. Check this option when submitting a revised (altered or corrected) or amended application. See also the NIH Application Submission Policies. If your application is both a "New/Revision/Renewal" and a "Resubmission," check only the "Resubmission" box.
- Renewal. Check this option if you are requesting additional funding for a period subsequent to that provided by a current award. A renewal application competes with all other applications and must be developed as fully as if the applicant were applying for the first time.
- Continuation. The box for "Continuation" is used only for specific FOAs.
- Revision. Check this option for competing revisions and non-competing administrative supplements. For more information on competing revisions, see NIH Competing Revisions. For more information on administrative supplements, see NIH Administrative Supplements.
Additional Instructions for Career Development:
The applicant should generally check "New" or "Resubmission." Unless otherwise specified in the FOA, individual career development awards usually cannot be renewed, supplemented, or revised. Contact the awarding component staff or refer to the FOA if clarification is needed.
Additional Instructions for Fellowship:
The applicant should generally check "New" or "Resubmission." Unless otherwise specified in the FOA, individual fellowship awards usually cannot be renewed, supplemented, or revised. Contact the awarding institute or center staff or refer to the FOA if clarification is needed.
Additional Instructions for SBIR/STTR:
For more information about SBIR/STTR application types, see the SBIR/STTR Frequently Asked Questions.
If Revision, mark appropriate box(es).
You may select more than one.
- Increase Award
- Decrease Award
- Increase Duration
- Decrease Duration
- Other (specify)
If "E. Other (specify)" is selected, specify in the space provided.
The boxes for options B, C, D, and E will generally not be used and should not be selected unless specifically addressed in a particular FOA.
Is this application being submitted to other agencies? What Other Agencies?
In the field "Is this application being submitted to other agencies?" check "Yes" if one or more of the specific aims submitted in your application is also contained in a similar, identical, or essentially identical application submitted to another federal agency.
Otherwise, check "No."
If you checked "Yes," indicate the agency or agencies to which the application has been submitted.
9. Name of Federal Agency
The "Name of Federal Agency" field is pre-populated from the opportunity package and reflects the agency from which assistance is being requested with this application.
10. Catalog of Federal Domestic Assistance Number and Title
This field is pre-populated from the opportunity package and reflects the Catalog of Federal Domestic Assistance (CFDA) number of the program under which assistance is requested.
This field may be blank if you are applying to an opportunity that references multiple CFDA numbers. When this field is blank, leave it blank. The appropriate CFDA number will be automatically assigned by the agency once the application is assigned to the appropriate awarding component.
11. Descriptive Title of Applicant's Project
This field is required.
Additional Instructions for Multi-project:
Other Components: The "Descriptive Title of Applicant's Project" section is required.
Enter a brief descriptive title of the project.
The descriptive title is limited to 200 characters, including spaces and punctuation.
New Applications: You must have a title different than any other NIH or other PHS Agency project submitted for the same application due date with the same Project Director/Principal Investigator (PD/PI).
Resubmission or Renewal Applications: You should normally have the same title as the previous grant or application; however, if the specific aims of the project have significantly changed, choose a new title.
Revision Applications: You must have the same title as the currently funded grant.
Additional Instructions for SBIR/STTR:
An SBIR/STTR Phase II application should have the same title as the previously awarded Phase I grant.
12. Proposed Project
Additional Instructions for Multi-project:
Other Components: The "Proposed Project" section is required.
Start Date:
This field is required. Enter the proposed start date of the project. The start date is an estimate, and is typically at least nine months after application submission. The project period should not exceed what is allowed in the FOA.
Additional Instructions for Training:
The usual start date for an institutional training grant is July 1, but there are other possible start dates. Refer to the Table of IC-Specific Information, Requirements and Staff Contacts in your FOA or contact the awarding component staff for further information.
Ending Date:
This field is required. Enter the proposed ending date of the project.
Additional Instructions for SBIR/STTR:
Phase I: Routinely, SBIR Phase I awards do not exceed six months, and STTR Phase I awards do not exceed one year.
Phase II and Commercialization Readiness Pilot (CRP): Routinely, both SBIR and STTR Phase II awards do not exceed two years.
Under special circumstances, applicants to NIH may propose longer periods of time for completion of the research project (e.g., feasibility demonstration). Such requests must be thoroughly justified. Project duration deviations apply to NIH only, as CDC, FDA, and ACF do not make awards for periods longer than the stated guidelines.
13. Congressional District of Applicant
Enter the Congressional District as follows: a 2-character state abbreviation, a hyphen, and a 3-character district number. Examples: CA-005 for California's 5th district, VA-008 for Virginia's 8th district.
If outside the United States, enter 00-000.
For States and U.S. Territories with only a single congressional district, enter "001" for the district number.
For jurisdictions with no representative, enter "099."
For jurisdictions with a nonvoting delegate, enter "098" for the district number. Example: DC-098 or PR-098.
If you do not know your Congressional District: Go to The United States House of Representatives website and search for your Congressional District by entering your ZIP+4. If you do not know your ZIP+4, look it up on the USPS Look Up Zip Code website.
14. Project Director/Principal Investigator Contact Information
This information is for the PD/PI. The PD/PI is the individual responsible for the overall scientific and technical direction of the project.
In the eRA Commons profile, the person listed here in "14. Project Director/Principal Investigator Contact Information" must be affiliated with the applicant organization entered in "5. Applicant Information." If you are proposing research at an institute other than the one you are currently at, do not create a separate Commons account with the proposed applicant organization. For additional information on creating affiliations for users in the eRA Commons, see eRA Account Management System's Online Help.
If submitting an application reflecting multiple PD/PIs, the individual listed here as the Contact PD/PI in "14. Project Director/Principal Investigator Contact Information" will be the first PD/PI listed in G.240 - R&R Senior/Key Person Profile (Expanded) Form.
See G.240 - R&R Senior/Key Person Profile (Expanded) Form for additional instructions for multiple PD/PIs. To avoid potential errors and delays in processing, ensure that the information provided in this section is identical to the PD/PI profile information contained in the eRA Commons.
Additional Instructions for Career Development:
Provide the name of the individual candidate (considered the PD/PI for career development award programs). If the PD/PI is not located at the applicant organization at the time the application is submitted, the information should reflect where the candidate can be reached prior to the requested award start date. If the PD/PI is not located at the applicant organization at the time of submission, the Commons account for the PD/PI must be affiliated with the applicant organization.
If your proposed career award is at a different site than your current institution, the proposed sponsoring institution will be the applicant organization. You must affiliate your Commons account with the institution so that you have access to records submitted on your behalf. Do not create a separate Commons account with the proposed sponsoring institution.
Note: For some career transition award programs (e.g., K22) the applicant may apply without an institutional affiliation. These individuals should refer to the specific FOA for application instructions.
Multiple PD/PIs cannot apply for individual career development awards.
Additional Instructions for Fellowship:
Provide the name of the individual fellowship applicant (considered the PD/PI for fellowship award programs). If the PD/PI is not located at the applicant organization at the time the application is submitted, the information should reflect where the fellowship applicant can be reached prior to the requested award start date.
If your proposed fellowship is at a different site than your current institution, the proposed sponsoring institution will be the applicant organization. You must affiliate your Commons account with the institution so that you have access to records submitted on your behalf. Do not create a separate Commons account with the proposed sponsoring institution.
Multiple PD/PIs cannot apply to fellowship applications.
Additional Instructions for SBIR/STTR:
For Single PD/PI Applications: Name the one person responsible to the applicant small business concern (SBC) for the scientific and technical direction of the project in the "14. PD/PI Contact Information" section.
For Multiple PD/PI Applications: Name the contact PD/PI here in "14. PD/PI Contact Information." The Contact PD/PI (as designated here in "14. PD/PI Contact Information") must be listed first in the G.240 - R&R Senior/Key Person Profile (Expanded) Form and must be affiliated with the applicant organization in the PD/PI's eRA Commons profile.
NIH and PHS staff conduct official business only with the named PD/PIs and organizational/institutional officials.
A revision/supplemental application must have the same contact PD/PI as the currently funded grant.
SBIR
Phase I, Phase II, and CRP: The primary employment of the PD/PI must be with the SBC at the time of award and during the conduct of the proposed project. Primary employment means that more than one half (greater than 50%) of the PD/PI's time is spent in the employ of the SBC. Primary employment with an SBC precludes full-time employment at another organization. Occasionally, deviations from this requirement may occur. Such deviations must be approved in writing by the grants management officer after consultation with the NIH SBIR/STTR Program Coordinator.
Phase I, Phase II, and CRP Multiple PD/PI applications: The PD/PI listed here in "14. PD/PI Contact Information" must be affiliated with the applicant SBC organization submitting the application and will serve as the contact PD/PI. The primary employment of the "Contact PD/PI" must be with the SBC at the time of award and during the conduct of the proposed project. As noted above, occasionally, deviations from this requirement may occur. Such deviations must be approved in writing by the grants management officer after consultation with the NIH SBIR/STTR Program Coordinator.
PD/PI Definition: As defined in 42 CFR 52, the PD/PI(s) is or are the "...individual(s) judged by the applicant organization to have the appropriate level of authority and responsibility to direct the project or program supported by the grant and who is or are responsible for the scientific and technical direction of the project." When the proposed PD/PI clearly does not have sufficient qualifications to assume this role, the application is not likely to receive a favorable evaluation.
Verification of PD/PI Eligibility: If the application has the likelihood for funding, the awarding component will require documentation to verify the eligibility of the PD/PI, if at the time of submission of the application, the PD/PI meets any of the following criteria:
- is a less-than-full-time employee of the SBC;
- is concurrently employed by another organization;
- gives the appearance of being concurrently employed by another organization, whether for a paid or unpaid position.
If the PD/PI is employed or appears to be employed by an organization other than the applicant organization in any capacity (such as Research Fellow, Consultant, Adjunct Professor, Clinical Professor, Clinical Research Professor, or Associate), a letter must be provided by each employing organization confirming that, if an SBIR grant is awarded to the applicant SBC, the PD/PI is or will become a less-than-half-time employee of such organization and will remain so for the duration of the SBIR project. If the PD/PI is employed by a university, such a letter must be provided by the Dean's office or equivalent; for other organizations, the letter must be signed by a corporate official.
This requirement applies also to those individuals engaged currently as the PD/PI on an active SBIR project. All current employment and all other appointments of the PD/PI must be identified in his or her "Biographical Sketch" required as part of the application. Be certain that correct beginning and ending dates are indicated for each employment record listed.
STTR
Phase I and Phase II: The primary employment of the principal investigator must be with the SBC or the research institution at the time of award and during the conduct of the proposed project. Primary employment means that more than one half (greater than 50%) of the PD/PI's time is spent in the employ of the SBC or the research institution. Primary employment with an SBC or research institution precludes full-time employment at another organization. An SBC may replace the principal investigator on an STTR Phase I or Phase II award, subject to approval in writing by the Funding Agreement Officer. For purposes of the STTR Program, personnel obtained through a Professional Employer Organization or other similar personnel leasing company may be considered employees of the awardee. This is consistent with SBA's size regulations, 13 CFR 121.106-Small Business Size Regulations.
For Multiple PD/PI Applications: The PD/PI listed here in "14. PD/PI Contact Information" must be affiliated with the applicant SBC submitting the application and will serve as the Contact PD/PI. The Contact PD/PI may be from either the SBC or the single partnering research institution.
Note: The Contact PD/PI must have a formal appointment with or commitment to the SBC, which must be in the form of an official relationship between the parties, but need not include a salary or other form of remuneration.
PD/PI Eligibility: The PD/PI must commit a minimum of 10% (1.2 calendar months) effort to the project and must have a formal appointment with or commitment to the applicant SBC, which is characterized by an official relationship between the SBC and that individual. Such a relationship does not necessarily involve a salary or other form of remuneration. In all cases, however, the PD/PI's official relationship with the grantee must entail sufficient opportunity for the PD/PI to carry out his or her responsibilities for the overall scientific and technical direction of the project. Although documentation (e.g., consortium and contractual arrangements) describing the official relationship of the PD/PI with the applicant SBC should NOT be submitted with the grant application, a copy must be furnished upon the request of the NIH awarding component.
Following is guidance for such documentation (describing the official relationship of the PD/PI with the applicant SBC), which is required prior to award. The letter should be prepared on the letterhead of the independent PD/PI and addressed to the SBC. One page is recommended. At a minimum, the letter should (1) verify the PD/PI's commitment to the project; (2) refer to the specific project by name; and (3) specify what assets or services the PI will contribute (e.g. expertise, number of hours/percent effort) as well as the PD/PI's remuneration. The letter should also indicate that the PD/PI and the SBC have reached an agreement on proprietary interests (e.g., intellectual property).
Signatures of the authorized organization representative (AOR or signing official) for the applicant organization on the Authorized Representative section and the signature of the duly authorized representative of the research institution certifies, among other things, that the PD/PI has a formal relationship with/commitment to the SBC when the PD/PI is an employee of the Research Institute.
The following are examples of situations describing the official relationship of the PD/PI with the applicant small business organization:
- PD/PI with a full-time, university appointment may also have appointments with other organizations (with or without salary) and still appropriately consider his or her commitment to the university to be "full-time," consistent with the personnel policies and procedures of the university applied on a routine basis. The PD/PI's commitment to the university and other organizations (including the applicant SBC) cannot exceed 100% of his or her total professional effort.
- PD/PI with a full-time, 12-month appointment with an SBC would be considered to have a commitment to the applicant organization of 100% of his or her total professional effort.
- PD/PI who has a part-time appointment with an SBC and has concurrent commitments or appointments with organizations in addition to the small business concern would deem each commitment as a portion of 100% of his or her total professional effort.
As responsible stewards of funds, the NIH is concerned that the PD/PI has the time available to carry out the proposed STTR research activities. Therefore, it should be clear in the application that the time proposed for the PD/PI on a particular project is reasonable and it should be clear that the PD/PI has sufficient time (minimum 10% effort, which is 1.2 calendar months) from among his or her total professional commitments to devote to this project.
Prefix:
Enter or select the prefix, if applicable, for the name of the PD/PI.
First Name:
This field is required. Enter the first (given) name of the PD/PI.
Middle Name:
Enter the middle name of the PD/PI.
Last Name:
This field is required. Enter the last (family) name of the PD/PI.
Suffix:
Enter or select the suffix, if applicable, for the PD/PI. Do not use this field to record degrees (e.g., Ph.D. or M.D.). Degrees for the PD/PI are requested separately in the R&R Senior/Key Person Profile (Expanded) Form.
Position/Title:
Enter the position/title of the PD/PI.
Organization Name:
This field is required. This field may be pre-populated from the applicant information section in this form.
Department:
Enter the name of primary organizational department, service, laboratory, or equivalent level within the organization of the PD/PI.
Division:
Enter the name of primary organizational division, office, major subdivision, or equivalent level within the organization of the PD/PI.
Street1:
This field is required. Enter first line of the street address for the PD/PI.
Street2:
Enter the second line of the street address for the PD/PI.
City:
This field is required. Enter the city for the address of the PD/PI.
County/Parish:
Enter the county/parish for the address of the PD/PI.
State:
This field is required if the PD/PI is located in the United States or its Territories. Enter the state or territory where the PD/PI is located.
Province:
If "Country" is Canada, enter the province for the PD/PI; otherwise, skip the "Province" field.
Country:
Select the country for the PD/PI.
ZIP/Postal Code:
The ZIP+4 is required if the PD/PI address is in the United States. Otherwise, the postal code is optional. Enter the ZIP+4 (nine-digit postal code) or postal code of the PD/PI.
Phone Number:
This field is required. Enter the daytime phone number for the PD/PI.
Fax Number:
Enter the fax number for the PD/PI.
E-mail:
This field is required. Enter the e-mail address for the PD/PI.
15. Estimated Project Funding
All four fields in "15. Estimated Project Funding" are required.
a. Total Federal Funds Requested
Enter the total federal funds, including Direct Costs and F&A Costs (Indirect Costs), requested for the entire project period.
Additional Instructions for Fellowship:
Applicants should refer to the NIH Research Training and Career Development website for current stipend and other budgetary levels. Enter the total amount requested for the entire period of support. This amount should include the applicable stipend amount, the actual tuition and fees, and the standard institutional allowance.
If new stipend or other payment levels for Kirschstein-NRSA fellowships are announced after the time of application, these amounts will be automatically adjusted at the time of award.
Extraordinary Costs: Additional funds may be requested by the institution when the training of a fellow involves extraordinary costs for travel to field sites remote from the sponsoring institution or accommodations for fellows who are disabled, as defined by the Americans with Disabilities Act. The funds requested for extraordinary costs must be reasonable in relationship to the total dollars awarded under a fellowship and must be directly related to the approved research training project. Such additional funds shall be provided only in exceptional circumstances that are fully justified and explained by the institution in the application.
Additional Instructions for SBIR/STTR:
Enter total federal funds, including Direct Costs, F&A Costs (Indirect Costs), and Fee, requested for the entire project period.
According to statutory guidelines, total funding support (direct costs, indirect costs, fee) normally may not exceed $150,000 for Phase I awards and $1,000,000 for Phase II awards. With appropriate justification from the applicant, Congress will allow awards to exceed these amounts by up to 50% ($225,000 for Phase I and $1,500,000 for Phase II, a hard cap). As written in the statute and under appropriate circumstances, NIH can apply for a waiver from SBA to issue an award exceeding $225,000 for Phase I or $1,500,000 for Phase II, if this hard cap will interfere with NIH's ability to meet its mission. Award waivers from the SBA are not guaranteed and may delay the release of funds. Applicants are strongly encouraged to contact NIH program officials prior to submitting any award in excess of the guidelines. In all cases, applicants should propose a budget that is reasonable and appropriate for completion of the research project. Note: CDC, FDA, and ACF do not make awards above these statutory guidelines.
b. Total Non-Federal Funds
For applications to NIH and other PHS agencies, enter "0" in this field unless cost sharing is a requirement for the specific FOA.
c. Total Federal & Non-Federal Funds
Enter the total federal and non-federal Funds requested. The amount in this field will be the same as the amount in the "Total Federal Funds Requested" field unless the specific FOA indicates that cost sharing is a requirement.
d. Estimated Program Income
Indicate any program income estimated for this project, if applicable.
Additional Instructions for Training:
Enter "0," as the "Estimated Program Income" does not apply to training applications.
Additional Instructions for Fellowship:
Enter "0," as the "Estimated Program Income" does not apply to fellowship applications.
16. Is Application Subject to Review by State Executive Order 12372 Process?
Applicants should check "No, Program is not covered by E.O. 12372."
17. Certification
This field is required.
The list of NIH and other PHS agencies Certifications, Assurances, and other Policies is found in the NIH Grants Policy Statement, Section 4: Public Policy Requirements and Objectives.
The applicant organization is responsible for verifying its eligibility and the accuracy, validity, and conformity with the most current institutional guidelines of all the administrative, fiscal, and scientific information in the application, including the Facilities and Administrative rate. Deliberate withholding, falsification, or misrepresentation of information could result in administrative actions, such as withdrawal of an application, suspension and/or termination of an award, debarment of individuals, as well as possible criminal and/or civil penalties. The signer further certifies that the applicant organization will be accountable both for the appropriate use of any funds awarded and for the performance of the grant-supported project or activities resulting from this application. The grantee institution may be liable for the reimbursement of funds associated with any inappropriate or fraudulent conduct of the project activity.
Check "I agree" to provide the required certifications and assurances.
18. SFLLL (Disclosure of Lobbying Activities) or Other Explanatory Documentation
If applicable, attach the SFLLL or other explanatory document as per FOA instructions.
If unable to certify compliance with the Certification in the "17. Certification" section above, attach an explanation. Additionally, as applicable, attach the SFLLL (Standard Form LLL, Disclosure of Lobbying Activities) or other documents in this item.
For more information:
See the NIH Grants Policy Statement, Section 4.1.17: Lobbying Prohibition, and the NIH Lobbying Guidance for Grantee Activities page.
19. Authorized Representative
The authorized representative is equivalent to the individual with the organizational authority to sign for an application. This individual is otherwise known as the authorized organization representative (AOR) in Grants.gov or the signing official (SO) in eRA Commons.
Prefix:
Enter or select the prefix, if applicable, for the name of the AOR/SO.
First Name:
This field is required. Enter the first (given) name of the AOR/SO
Middle Name:
Enter the middle name of the AOR/SO.
Last Name:
This field is required. Enter the last (family) name of the AOR/SO.
Suffix:
Enter or select the suffix, if applicable, for the AOR/SO.
Position/Title:
This field is required. Enter the position/title of the name of the AOR/SO.
Organization Name:
This field is required. Enter the name of the organization for the AOR/SO.
Department:
Enter the name of the primary organizational department, service, laboratory, or equivalent level within the organization for the AOR/SO.
Division:
Enter the name of the primary organizational division, office, major subdivision, or equivalent level within the organization for the AOR/SO.
Street1:
This field is required. Enter the first line of the street address for the AOR/SO.
Street2:
Enter the second line of the street address for the AOR/SO.
City:
This field is required. Enter the city for the address of the AOR/SO.
County/Parish:
Enter the county/parish for the address of the AOR/SO.
State:
This field is required if the AOR/SO is located in the United States or its Territories. Enter the state or territory where the AOR/SO is located.
Province:
If "Country" is Canada, enter the province for the AOR/SO; otherwise, skip the "Province" field.
Country:
Select the country for the address of the AOR/SO.
ZIP/Postal Code:
The ZIP+4 is required if the AOR/SO is in the United States. Otherwise, the postal code is optional Enter the ZIP+4 (nine-digit postal code) or postal code of the AOR/SO.
Phone Number:
This field is required. Enter the daytime phone number for the AOR/SO.
Fax Number:
Enter the fax number for the AOR/SO.
Email:
This field is required. Enter the e-mail address for the AOR/SO.
Signature of Authorized Representative:
Grants.gov will record the electronic signature for the AOR/SO who submits the application.
It is the organization's responsibility to assure that only properly authorized individuals sign in this capacity and/or submit the application to Grants.gov.
Date Signed:
Grants.gov will generate this date upon application submission.
20. Pre-application
Unless specifically noted in a FOA, NIH and other PHS agencies do not use pre-applications. The "Pre-application" attachment field should not be used for any other purpose.
If permitted by your FOA, attach this information as a PDF.
21. Cover Letter Attachment
The cover letter is for internal use only and will not be shared with peer reviewers.
Who must complete the "Cover Letter Attachment:"
Refer to the "content" list below for items that are permitted, as well as for specific situations in which a cover letter must be included.
A cover letter must not be included with post-award submissions, such as administrative supplements, change of grantee institution, or successor-in-interest.
Format:
Attach the cover letter, addressed to the Division of Receipt and Referral, in accordance with the FOA and/or these instructions.
Attach the cover letter in the correct location, specifically verifying that the cover letter has not been uploaded to the "20. Pre-application" field which is directly above the "21. Cover Letter Attachment" field. This will ensure the cover letter attachment is kept separate from the assembled application in the eRA Commons and made available only to appropriate staff.
Content:
The letter should contain any of the following information, as applicable:
- Application title.
- Title of FOA (PA or RFA).
- For late applications (see Late Application policy on NIH's Application Submission Policies) include specific information about the timing and nature of the delay.
- For changed/corrected applications submitted after the due date, a cover letter is required, and it must explain the reason for late submission of the changed/corrected applications. If you already submitted a cover letter with a previous submission and are now submitting a late change/corrected application, you must include all previous cover letter text in the revised cover letter attachment. The system does not retain any previously submitted cover letters; therefore, you must repeat all information previously submitted in the cover letter as well as any additional information.
- Explanation of any subaward budget components that are not active for all budget periods of the proposed grant (see G.310 - R&R Subaward Budget Attachment(s) Form).
- Statement that you have attached any required agency approval documentation for the type of application submitted. This may include approval for applications that request $500,000 or more, approval for Conference Grant or Cooperative Agreement (R13 or U13), etc. It is recommended that you include the official communication from an NIH official as part of your cover letter attachment.
- When intending to submit a video as part of the application, the cover letter must include information about the intent to submit it; if this is not done, the video will not be accepted. See NIH Grants Policy Statement, Section 2.3.7.7: Post Submission Grant Application Materials for additional information.
- Include a statement in the cover letter if the proposed studies will generate large-scale human or non-human genomic data as detailed in the NIH Genomic Data Sharing Policy (see the NIH Grants Policy Statement, Section 2.3.7.10: NIH Genomic Data Sharing and Section 8.2.3.3: Genomic Data Sharing (GDS) Policy/Policy for Genome-Wide Association Studies (GWAS)).
Additional Instructions for Career Development:
Mentored Career Development Award (CDA) applicants must include a cover letter that contains a list of referees (including name, departmental affiliation, and institution).
Non-mentored CDA applicants are encouraged, but not required, to include a cover letter. The cover letter should include a list of referees (including name, departmental affiliation, and institution).
Additional Instructions for Fellowship:
Individual fellowship applicants must include a cover letter that contains a list of referees (including name, departmental affiliation, and institution).
Additional Instructions for SBIR/STTR:
If Phase I or Phase II was a contract or awarded from another federal agency, include the contract or award number.
