U.S. Food and Drug Administration (FDA)
NOTE: The policies, guidelines, terms, and conditions stated in this announcement may differ from those used by the NIH. Where this Funding Opportunity Announcement (FOA) provides specific written guidance that may differ from the general guidance provided in the grant application form, please follow the instructions given in this FOA.
The FDA does not follow the NIH Page Limitation Guidelines or the NIH Review Criteria. Applicants are encouraged to consult with FDA Agency Contacts for additional information regarding page limits and the FDA Objective Review Process.
Office of Regulatory Affairs (ORA)
Flexible Funding Model - Infrastructure Development and Maintenance for State Manufactured Food Regulatory Programs (U18)
U18 Research Demonstration Cooperative Agreements
New
- January 20, 2022 - RFA-FD-22-019
- November 04, 2019 - Intent to Publish Notice: FSMA Preventive Controls and Mutual Reliance Implementation Expansion Supplement to RFA-FD-18-001 NOT-FD-20-002.
- October 30, 2019 - FSMA Preventive Controls and Mutual Reliance Implementation Expansion Supplement to RFA-FD-18-001. See Notice NOT-FD-20-001.
- August 23, 2019 - Clarifying Competing Application Instructions and Notice of Publication of Frequently Asked Questions (FAQs) Regarding Proposed Human Fetal Tissue Research. See Notice NOT-OD-19-137.
- July 26, 2019 - Changes to NIH Requirements Regarding Proposed Human Fetal Tissue Research. See Notice NOT-OD-19-128.
RFA-FD-18-001
93.367
The intended outcome of this FOA is to advance efforts for a nationally integrated food safety system (IFSS) by supporting Manufactured Food Regulatory Program Standards (MFRPS), Rapid Response Teams (RRT) and Food Protection Task Force (FPTF) programs, as well as special projects. For the purposes of this FOA, the term State encompasses all eligible organizations as defined in Section 3.
The purpose of this FOA section is to advance efforts for a nationally integrated food safety system by assisting State manufactured food regulatory programs to achieve and maintain conformance with the most current version of the Manufactured Food Regulatory Program Standards (MFRPS). The MFRPS are intended to ensure that State manufactured food regulatory programs develop and maintain best practices for a high-quality regulatory program. Also, the program standards are intended to enhance food safety by establishing a uniform basis for measuring and improving the performance of manufactured food regulatory programs in the United States. Conformance with these program standards will help Federal and State programs better direct their regulatory activities at reducing foodborne illness hazards in plants that manufacture, process, pack, or hold foods.
The purpose of this funding option is to establish and/or support a Food Protection Task Force responsible for promoting the integration of an efficient statewide human and animal food (HAF) protection system that maximizes the protection of the public health. These efforts would include: fostering communication, education, outreach, cooperation and collaboration within the states among federal, state, local, tribal and territorial HAF protection, public health, agriculture, and regulatory agencies, industry, academia, and consumers to initiate and/or support HAF protection activities to improve public health.
The purpose of this FOA section is to facilitate long-term improvements and innovation to the national integrated food safety system by unifying and coordinating federal/state/local HAF emergency response efforts including:
1) Strengthening the link among epidemiology, lab and environmental health/regulatory components;
2) Improving States' regulatory and surveillance HAF protection programs to include using Incident Command System (ICS)/National Incident Management System (NIMS) principles and a Unified Command structure to conduct integrated responses to all-hazards HAF emergencies, rapidly identifying and removing tainted food from commerce, and conducting root cause investigations to inform future prevention efforts; and
3) Addressing supporting components, such as training, data sharing, data analysis, communications, continuous process improvement, and development of best practices and other resources to support national capacity/capability development.
The purpose of this FOA section is to develop and implement special projects that support innovation and integration in a nationally Integrated Food Safety System (IFSS) using the MFRPS or RRT framework. State programs will be expected to share project deliverables and resources developed with other programs.
October 31, 2017
November 6, 2017
November 22, 2017
March 1, 2018, by 11:59 PM Eastern Time.
December 1, 2018, by 11:59 PM Eastern Time.
December 1, 2019, by 11:59 PM Eastern Time.
December 1, 2020, by 11:59 PM Eastern Time.
December 1, 2021, by 11:59 PM Eastern Time.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
Applicants should be aware that on-time submission means that an application is submitted error free (of both Grants.gov and eRA Commons errors) by 11:59 PM Eastern Time on the application due date.
Late applications will not be accepted for this FOA.
Not Applicable
April, 2018
February, 2019
February, 2020
February, 2021
Not Applicable
August 1, 2018
June 1, 2019
June 1, 2020
June 1, 2021
June 1, 2022
December 2, 2021
Not Applicable
It is critical that applicants follow the instructions in the SF424 (R&R) Application Guide, except where instructed to do otherwise (in this FOA or in a Notice from the NIH Guide for Grants and Contracts). Conformance to all requirements (both in the Application Guide and the FOA) is required and strictly enforced. Applicants must read and follow all application instructions in the Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the Application Guide, follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
Part 1. Overview Information
Part 2. Full Text of the Announcement
Section
I. Funding Opportunity Description
Section II. Award Information
Section III. Eligibility Information
Section IV. Application and Submission
Information
Section V. Application Review Information
Section VI. Award Administration Information
Section VII. Agency Contacts
Section VIII. Other Information
This cooperative agreement is intended to support the Manufactured Food Regulatory Program Standards (MFRPS), Rapid Response Teams (RRT) and Food Protection Task Force (FPTF) Programs, as well as Special Projects supporting these programs. Applicants must refer to the Eligibility Section of this FOA to determine which funding track they should apply for: MFRPS Development or MFRPS Maintenance. Within each track, applicants applying for more than one funding option should include activities supporting the appropriate funding options, as outlined below. Further information on eligibility for each of the funding options, maximum budget per program area and other administrative considerations can be found in this document below.
A schematic outlining the two funding tracks and the associated funding options available for each track is below:
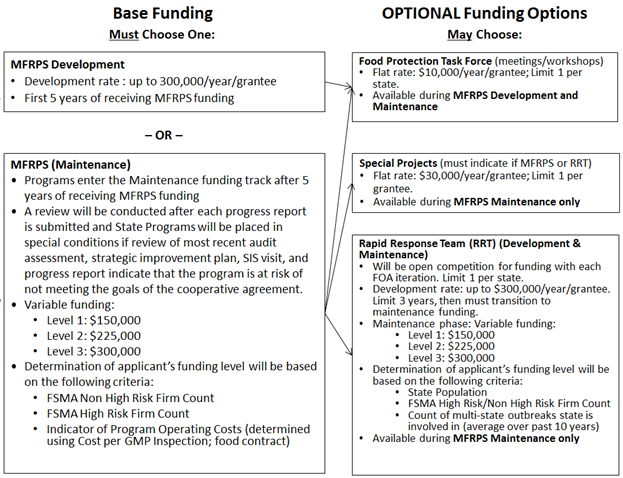
Applicants should take sustainability into account when designing projects proposed under this cooperative agreement to maximize the longevity of resulting outcomes, resources and program infrastructure beyond the end of the project period.
1. MFRPS (Required - either Development or Maintenance)
The Manufactured Food Regulatory Program Standards (MFRPS) allows for the development of risk-based food safety programs by establishing a uniform basis for measuring and improving the performance of State manufactured food regulatory programs in the United States.
For more information on the MFRPS Program, please visit http://www.fda.gov/ForFederalStateandLocalOfficials/ProgramsInitiatives/RegulatoryPrgmStnds/ucm475064.htm.
For the purpose of this funding opportunity, conformance means the fulfillment of a requirement of the MFRPS. Specifically a State program is using and can demonstrate the use of a particular element, system, or program listed in the MFRPS (most recent version).
Although individual year outcomes may vary among programs, the overall outcomes of the work provided under this cooperative agreement are as follows:
(1) Grantees will achieve and maintain conformance with the MFRPS (most recent version). If conformance is not achieved by the conclusion of the cooperative agreement, the state will have a strategic improvement plan to reach conformance.
(2) Grantees will contribute to the continuous improvement of the MFRPS through attendance at an annual face-to-face meeting, active participation in committees, and other initiatives.
(3) Grantees will develop strategies and resources for achieving and maintaining conformance with the MFRPS that can be shared and duplicated on a national basis.
(4) Grantees will provide FDA the foundation for pursuing regulatory action based upon the findings of State manufactured food regulatory programs. Grantees will provide FDA the foundation to improve quality of contracts, coordination of inspections, investigations and enforcement to effectively and efficiently protect public health.
(5) If the grantee is located in a state with a supporting laboratory that receives funding under an active FDA cooperative agreement for maintaining and/or expanding ISO 17025 accreditation for analysis of human food, the regulatory program grantee will provide for the collection of samples (FDA regulated products only) to support laboratory capacity development and product surveillance. The applicant must also demonstrate the ability to perform any enforcement or other follow-up activities based on sample results. Sampling plans will be developed in cooperation with the laboratory to support the objectives of both programs.
2. RRT (Optional)
The Rapid Response Teams (RRT) Program supports development and maintenance/continued operations of multi-jurisdictional, multi-disciplinary Rapid Response Teams (RRTs) for program improvement and requires extensive cooperation and coordination among State programs (food and feed regulatory, laboratory, epidemiology, emergency management) and their corresponding FDA District Office. Incident response and surveillance work conducted by the RRT, including sample collection and laboratory analyses, are considered within scope for this funding option.
For more information on the RRT Program, please visit http://www.fda.gov/ForFederalStateandLocalOfficials/ProgramsInitiatives/ucm475021.htm.
The yearly goals outlined for the RRT funding option are in alignment with the RRT Program 5 Year Plan and the RRT Capacity Building Process & Mentorship Framework. These documents are available via FoodSHIELD for applicants with prior involvement in the RRT Program, or by emailing [email protected].
Yearly Goals for RRT Development and RRT Maintenance:
The yearly goals for RRT Development and Maintenance are available via FoodSHIELD for applicants with prior involvement in the RRT Program, or by emailing [email protected]. Applicants must review and address these yearly goals within their application.
3. FPTF (Optional)
FDA views State-based Food Protection Task Forces as an important mechanism for providing food protection program coordination and information exchange within each State. ("Food" includes human food and animal food and is defined in 21 USC 321(f)). Funding is available to eligible state agencies to support an existing Food Protection Task Force, as well as to states that are in the process of developing a new Food Protection Task Force. Food Protection Task Force meetings should foster activities, communication, and cooperation among federal, state, local, and tribal public health and food safety agencies, industry, academia and consumers.
The Food Protection Task Force meetings/activities/projects should address the following objectives:
(1) Provide a forum for all the stakeholders of the state food protection system regulatory agencies, academia, industry, consumers, state legislators, Boards of Health and Agriculture, and other interested parties to improve HAF safety and defense;
(2) Assist in adopting or implementing applicable sections of the Code of Federal Regulations (CFR), the Food Code, and other food protection laws and regulations (e.g. Food Safety Modernization Act [FSMA], etc.); and
(3) Promote the concept of an Integrated Food Safety System (IFSS) by communicating and supporting agency and related federal, state, territorial, and tribal objectives that maximize the protection of the public health through integration activities including: inspections/investigations (coordination, process improvement), prevention (After Action Reviews, hot washes, etc. to identify areas for improvement, stakeholder training/education/outreach), intervention(coordination of compliance/enforcement and voluntary actions), response (multi-agency coordination, process improvements) and reviewing/discussing Partnership for Food Protection IFSS resources (integration best practices and recommendations- available at http://www.fda.gov/ForFederalStateandLocalOfficials/ProgramsInitiatives/PartnershipforFoodProtectionPFP/ucm404633.htm).
For more information on the FPTF Program, please visit http://www.fda.gov/ForFederalStateandLocalOfficials/ProgramsInitiatives/ucm475029.htm.
Yearly Goals for FPTF:
1. Hold at least one meeting with the FPTF stakeholders.
2. Identify an integration activity to address for each year of the cooperative agreement through the FPTF (see FPTF objective 3 above, "Promote the concept of an IFSS") and provide an update on the activity in the annual report.
3. Submit copies of FPTF presentations, job aids and other resources developed by the Task Force to program staff annually for sharing with other food protection stakeholders
4. Special Projects (Optional)
Projects that support innovation and integration in a nationally Integrated Food Safety System (IFSS) using the MFRPS or RRT framework. State programs will be expected to complete the special project within the proposed project period. All project deliverables and resources developed must be made available to other programs.
Examples of criteria to consider when developing special project proposals include:
a) Exceeds criteria in MFRPS program elements
b) Addresses an IFSS issue or problem
c) Is based on the most current public and environmental health and regulatory science and data available
d) Introduces an innovative approach (a new method, idea, protocol, tool/resource)
e) Produces outcome data and/or a product and lessons learned that can be shared with FDA and other stakeholders
f) Reflects and promotes inter-agency communication, collaboration, coordination, accountability, transparency and sharing of information
g) Supports FDA’s mission and vision for building mutual reliance in an IFSS under FSMA
h) Focuses on prevention, intervention or response activities
i) If response related (and applicant is also applying for RRT funding), exceeds yearly expectations/goals outlined in the RRT funding option
j) Supports national or external (outside of applicant's State) capacity/capability development for prevention, intervention or response
Potential special projects include but are not limited to:
- Development of a new inspections or investigations course, such as for a specific commodity, investigation technique, industry, or new rule
- Development of a new environmental sampling methodology to enhance efficiency
- Development of a system to collaborate with FDA and other agencies through IT information sharing
- Development of an electronic risk based modeling program that can demonstrate increased efficiency and effectiveness
- Development of a new foodborne illness prevention and/or intervention strategy designed for a specific targeted population.
- Development of best practices or piloting of innovative approaches/technology/tools related to inter-agency data sharing, communication and coordination during prevention, intervention and response.
- Evaluates, summarizes and shares lessons learned from using nationally recognized tools such as CIFOR Toolkit, PFP guidance documents and RRT Best Practices Manual with other state programs and stakeholders.
- Teaching and delivery of Preventive Controls Regulatory Course (FD 254) and other training courses to support FSMA implementation.
Applicants requesting funding for a Special Project must indicate whether the proposed project is a MFRPS or RRT-related special project in the application.
5. Legislation/other references of authority
National Integrated Food Safety System:
- http://www.fda.gov/ForFederalStateandLocalOfficials/ProgramsInitiatives/default.htm
- http://www.fda.gov/downloads/ForFederalStateandLocalOfficials/UCM183650.pdf
Food Safety Modernization Act:
- http://www.fda.gov/Food/GuidanceRegulation/FSMA/
- Full text of the law: http://www.fda.gov/food/guidanceregulation/fsma/ucm247548.htm
Consistent with the Uniform Guidance, codified at 2 CFR Part 200, an emphasis will be placed on the applicant’s ability to measure progress and track performance using objective, proven, and measurable data. As such, applicants will propose how they will develop and implement a performance measurement system, plan, and/or process and will carefully consider the Scored Review Criteria listed in Section V of this announcement when submitting their application.
See Section VIII. Other Information for award authorities and regulations.
Cooperative Agreement: A support mechanism used when there will be substantial Federal scientific or programmatic involvement. Substantial involvement means that, after award, FDA scientific or program staff will assist, guide, coordinate, or participate in project activities. See Section VI.2 for additional information about the substantial involvement for this FOA.
New
The OER Glossary and the SF424 (R&R) Application Guide provide details on these application types.
The number of awards and final funding levels is contingent upon FDA appropriations and the submission of a sufficient number of meritorious applications. Award(s) will provide one (1) year of support and include future recommended support for four (4) additional year(s) contingent upon annual appropriations, availability of funding and satisfactory awardee performance.
FDA/ORA intends to fund up to $18 million, for fiscal year 2018 in support of this grant program.
It is anticipated that up to fifty (50) awards will be made, not to exceed $640,000 in total costs (direct plus indirect), per award
Application budgets need to reflect the actual needs of the proposed project and should not exceed the following in total costs (direct and indirect). The Eligibility Section in this FOA outlines how to determine which funding track and associated options for which you may apply.
The below application budget limits are the same for all five (5) years of the cooperative agreement. Please note that the first year is less than twelve (12) months; see Award Project Period, directly below.
Funding Track 1: MFRPS Development:
MFRPS Base |
FPTF Option |
Potential Total Award (Up to) |
||||
$300,000 |
$10,000 |
$310,000 |
Level |
MFRPS Base |
FPTF Option |
Special Project Option |
RRT Dev. Option |
RRT Maint. Option |
Potential Total Award (Up to) |
1 |
$150,000 |
$10,000 |
$30,000 |
$300,000 |
$150,000 |
$460,000 |
2 |
$225,000 |
$10,000 |
$30,000 |
$300,000 |
$225,000 |
$565,000 |
3 |
$300,000 |
$10,000 |
$30,000 |
$300,000 |
$300,000 |
$640,000 |
The scope of the proposed project should determine the project period. The maximum project period is five (5) years. The first year of the award will be shortened (8/1/18 - 5/31/19). Future years' funding will be awarded for a 12 month budget period, see below.
Year 2: 6/1/19 - 5/31/20 (12 months)
Year 3: 6/1/20 - 5/31/21 (12 months)
Year 4: 6/1/21 - 5/31/22 (12 months)
Year 5: 6/1/22 - 5/31/23 (12 months)
HHS grants policies as described in the HHS Grants Policy Statement will apply to the applications submitted and awards made in response to this FOA.
Governments
- State Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- U.S. Territory or Possession, specifically the District of Columbia, Guam, the Commonwealth of Puerto Rico, the Northern Mariana Islands, the Virgin Islands, American Samoa, and the Trust Territory of the Pacific Islands.
This opportunity is only available to the following State, Tribal or Territorial food safety programs:
Manufactured food regulatory programs with current FDA food safety inspection contracts (providing funding to State agency food protection regulatory programs), or those that meet the eligibility requirements and agree to enter into a food safety inspection contract with FDA at the earliest possible date, are eligible to apply for funding under this cooperative agreement. Competition is limited to State, Tribal and Territorial manufactured food regulatory programs because the foundational work conducted under the current FDA food safety inspection contracts is necessary for the completion of significant improvements in a nationally integrated food safety system.
Applicants will be categorized under one of two funding tracks: MFRPS Development or MFRPS Maintenance (see eligibility criteria below). Within each funding track, an applicant may apply to additional funding options for which they meet the eligibility criteria (below).
Applicants are encouraged to apply for cooperative agreement assistance that reflects their jurisdiction’s needs both in terms of amounts of funding and number of project years.
To ascertain an applicant's tier’s funding ceiling, please consult Section II. Award Information.
Funding Track 1: MFRPS Development
MFRPS Development Funding Option (Funding Track 1)
Eligibility
- Received less than five (5) years funding under a past MFRPS cooperative agreement or have never received a MFRPS cooperative agreement.
- Applicants may request up to $300,000/year for this funding option.
- State programs will move to MFRPS Maintenance funding levels in the sixth year of funding under MFRPS cooperative agreements. Applicants with less than five (5) years funding under a past MFRPS cooperative agreement may elect to count years of MFRPS enrollment under the FDA Food Inspection Contract towards the five (5) years MFRPS funding eligibility requirement when determining the entry date for Funding Track 2 (MFRPS Maintenance).
- 2014 MFRPS Cooperative Agreement Awardees (PAR-13-164): State programs who began receiving MFRPS cooperative agreement funding in 2014 are only eligible to receive funding under Funding Track 1 for Year 1 of this cooperative agreement. For Years 2 - 5 of this cooperative agreement, these State programs must request funding under Funding Track 2 (MFRPS Maintenance).
- 2015 MFRPS Cooperative Agreement Awardees (PAR-13-164): State programs who began receiving MFRPS cooperative agreement funding in 2015 are only eligible to receive funding under Funding Track 1 for Years 1 and 2 of this cooperative agreement. For Years 3 - 5 of this cooperative agreement, these State programs must request funding under Funding Track 2 (MFRPS Maintenance).
- 2017 MFRPS Cooperative Agreement Awardees (RFA-FD-17-005): State programs who began receiving MFRPS cooperative agreement funding in 2017 are only eligible to receive funding under Funding Track 1 for Years 1-4 of this cooperative agreement. For Year 5 of this cooperative agreement, these State programs must request funding under the Funding Track 2 (MFRPS Maintenance).
- See below for MFRPS Funding Track 2 variable funding levels and additional funding options available in Funding Track 2.
- Programs who meet the eligibility requirements for Funding Track 1 (MFRPS Development):
- Are also eligible to apply for a FPTF option.
- Are not eligible to apply for RRT or Special Project funding options.
Food Protection Task Force (FPTF) Funding Option (Funding Track 1)
Eligibility
- State programs are eligible to apply for this option for both Funding Track 1 (MFRPS Development) and Funding Track 2 (MFRPS Maintenance). States with an existing Food Protection Task Force, as well as States that are developing a new Food Protection Task Force are both eligible to apply for this funding option. To apply for this funding option, a task force must provide a forum for all the stakeholders of the food protection system regulatory agencies, academia, industry, consumers, State legislators, Boards of Health and Agriculture, and other interested parties to improve food safety and defense.
- Applicants may request up to $10,000/year for this funding option.
- Only one application is allowed per state/territory. Jurisdictions interested in involving multiple state/territory entities (within their jurisdiction) are required to internally coordinate and choose one (1) entity to apply.
Funding Track 2: MFRPS Maintenance
MFRPS Maintenance Funding Option (Funding Track 2)
Eligibility
- The state program has received five (5) years funding under a past MFRPS cooperative agreement. Applicants with less than five (5) years funding under a past MFRPS cooperative agreement may elect to count years of MFRPS enrollment under the FDA Food Inspection Contract towards the five (5) years MFRPS funding eligibility requirement when determining the entry date for Funding Track 2 (MFRPS Maintenance).
- When a state program transitions from Funding Track 1 to Funding Track 2 during the course of the 5 year cooperative agreement (e.g., in Year 3), that state program also becomes eligible for the additional funding opportunities available under Funding Track 2 (such as: RRT or Special Projects).
- If the state program is not currently deemed to be in conformance and their corrective action is still pending (as documented in the most recent assessment by FDA/ORA/Office of Operations/Audit Staff), then the program must address the corrective action in their strategic improvement plan submitted as part of the application.
- Applicants have been classified into three (3) levels of funding ceilings based on a variety of factors unique to that jurisdiction. (See data sources below). This approach establishes funding ceilings proportional to the applicant’s jurisdictional work volume for this program area. To determine which funding level is applicable to your program, please consult the table below. Applicants are encouraged to apply for cooperative agreement assistance that reflects their jurisdiction’s needs both in terms of amounts of funding and number of project years.
*May not be eligible to select the MFRPS Maintenance Funding Option in Year 1 of the cooperative agreement (Funding Track 2); State program's eligibility to enter the MFRPS Maintenance Funding Track depends on date of first receiving a MFRPS Cooperative Agreement - see eligibility narrative for further details.
MFRPS Level 1 $150,000 |
MFRPS Level 2 $225,000 |
MFRPS Level 3 $300,000 |
Nebraska |
Alabama |
California - Health |
Nevada |
Alaska |
Florida |
South Carolina-Health |
Arkansas |
Illinois |
Vermont |
Colorado |
Michigan |
West Virginia-Agriculture |
Connecticut |
Minnesota |
West Virginia-Health |
Georgia |
New Jersey |
Indiana |
New York |
|
Iowa |
Ohio |
|
Mississippi* |
Kansas |
Oregon |
South Carolina - Agriculture* |
Kentucky |
Pennsylvania |
Wyoming* |
Maryland |
Texas |
Massachusetts |
Washington |
|
Missouri |
Wisconsin |
|
North Carolina |
||
Rhode Island |
||
Tennessee |
||
Utah |
||
Virginia |
||
Arizona* |
||
California - Agriculture* |
||
Idaho* |
||
Louisiana* |
||
Maine* |
||
Montana* |
||
New Mexico* |
||
Puerto Rico* |
||
*May not be eligible to select the MFRPS Maintenance Funding Option in Year 1 of the cooperative agreement (Funding Track 2); State program's eligibility to enter the MFRPS Maintenance Funding Track depends on date of first receiving a MFRPS Cooperative Agreement - see eligibility narrative for further details. |
||
- Data sources used to determine eligibility (see http://www.fda.gov/ForFederalStateandLocalOfficials/FundingOpportunities/GrantsCoopAgrmts/ucm539096.htm for further details):
- FY 18 FSMA High Risk Firm Count
- FY 18 FSMA Non High Risk Firm Count
- GMP inspection cost from the 2017-2018 food inspection contract year
- OP will maintain a copy of the funding algorithm and supporting data used to place State programs into funding levels. If you would like to obtain the data set specific to your State program, please email [email protected].
- Programs who meet the eligibility requirements for Funding Track 2 (MFRPS Maintenance):
- Are also eligible to apply for a FPTF option.
- Are also eligible to apply for RRT and Special Project funding options.
Rapid Response Team (RRT) Development Funding Option (Funding Track 2)
Eligibility
- The state program must apply for Funding Track 2: MFRPS Maintenance, demonstrate satisfactory progress towards the goals of the cooperative agreement, and not be in special condition status under the current award. The state program has received less than three (3) years funding under a past RRT cooperative agreement.
- Only one application is allowed per state/territory. Jurisdictions interested in involving multiple state/territory entities (within their jurisdiction) are required to internally coordinate and choose one (1) entity to apply.
- Eligibility is not limited to state programs who have participated in past RRT cooperative agreements.
- Applicants may request up to $300,000/year for this funding option for a maximum of 3 years. After receiving three (3) years of funding under the RRT Development Funding Option, the State program must transition to the RRT Maintenance Funding Option (which may result in a different funding level).
Rapid Response Team (RRT) Maintenance Funding Option (Funding Track 2)
Eligibility
- The state program must apply for Funding Track 2: MFRPS Maintenance, demonstrate satisfactory progress towards the goals of the cooperative agreement, and not be in special condition status under the current award. The state program has received three or more years funding under a past RRT cooperative agreement.
- Only one application is allowed per state/territory. Jurisdictions interested in involving multiple state/territory entities (within their jurisdiction) are required to internally coordinate and choose one (1) entity to apply.
- Applicants have been classified into three (3) levels of funding ceilings based on a variety of factors unique to that jurisdiction. (See data sources below). This approach establishes funding ceilings proportional to the applicant’s jurisdictional work volume for this program area. To determine which funding level is applicable to your program, please consult the table below. Applicants are encouraged to apply for cooperative agreement assistance that reflects their jurisdiction’s needs both in terms of amounts of funding and number of project years.
RRT Level 1 $150,000 |
RRT Level 2 $225,000 |
RRT Level 3 $300,000 |
Rhode Island |
Georgia |
California |
West Virginia |
Iowa |
Florida |
Maryland |
Michigan |
|
Massachusetts |
Minnesota |
|
Alaska* |
Missouri |
New York |
Idaho* |
North Carolina |
Pennsylvania |
Maine* |
Virginia |
Texas |
Mississippi* |
Washington |
|
Montana* |
||
Nebraska* |
Alabama* |
Illinois* |
Nevada* |
Arizona* |
New Jersey* |
New Mexico* |
Arkansas* |
Ohio* |
Vermont* |
Colorado* |
Wisconsin* |
Wyoming* |
Connecticut* |
|
Indiana* |
||
Kansas* |
||
Kentucky* |
||
Louisiana * |
||
Oregon* |
||
South Carolina* |
||
Tennessee* |
||
Utah* |
||
Puerto Rico* |
||
*May not be eligible to select the RRT Maintenance Funding Option in Year 1 of the cooperative agreement; State program's eligibility to select the RRT Maintenance Funding Track depends on when the State program is eligible for Funding Track 2 (MFRPS Maintenance Funding Option) and date of first receiving RRT funding - see eligibility narrative for further details. |
||
- Data sources used to determine eligibility (see http://www.fda.gov/ForFederalStateandLocalOfficials/FundingOpportunities/GrantsCoopAgrmts/ucm539096.htm for further details):
- State Population (2010 Census)
- FY 18 FSMA High Risk/Non High Risk Firm Count
- Count of multi-state outbreaks state is involved in (average over the ten year period of 2006-2015, CDC FOOD Tool)
- OP will maintain a copy of the funding algorithm and supporting data used to place State programs into funding levels. If you would like to obtain the data set specific to your State program, please email [email protected] or [email protected].
Food Protection Task Force (FPTF) Funding Option (Funding Track 2)
Eligibility
- State programs are eligible to apply for this option for both Funding Track 1 (MFRPS Development) and Funding Track 2 (MFRPS Maintenance).
- States with an existing Food Protection Task Force, as well as States that are developing a new Food Protection Task Force are both eligible to apply for this funding option.
- To apply for this funding option, a task force must provide a forum for all the stakeholders of the food protection system regulatory agencies, academia, industry, consumers, State legislators, Boards of Health and Agriculture, and other interested parties to improve food safety and defense.
- Applicants may request up to $10,000/year for this funding option.
- Only one award is allowed per state/territory. Jurisdictions interested in involving multiple state/territory entities (within their jurisdiction) are required to internally coordinate and choose one (1) entity to apply.
Special Projects Funding Option (Funding Track 2)
Eligibility
- The state program must apply for Funding Track 2: MFRPS Maintenance, demonstrate satisfactory progress towards the goals of the cooperative agreement, and not be in special condition status under the current award.
- Applicants must indicate in the budget narrative if the proposed project is a MFRPS or RRT-related special project.
- Applicants may request up to $30,000/year for this funding option.
Non-domestic (non-U.S.) Entities (Foreign Institutions) are
not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible
to apply.
Foreign components, as defined
in the HHS Grants Policy Statement, are not allowed.
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the SF 424 (R&R) Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission.
- Dun and Bradstreet Universal Numbering System (DUNS) - All registrations require that applicants be issued a DUNS number. After obtaining a DUNS number, applicants can begin both SAM and eRA Commons registrations. The same DUNS number must be used for all registrations, as well as on the grant application.
- System for Award Management (SAM) (formerly CCR) Applicants must complete and maintain an active registration, which requires renewal at least annually. The renewal process may require as much time as the initial registration. SAM registration includes the assignment of a Commercial and Government Entity (CAGE) Code for domestic organizations which have not already been assigned a CAGE Code.
- NATO Commercial and Government Entity (NCAGE) Code Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- eRA Commons - Applicants must have an active DUNS number and SAM registration in order to complete the eRA Commons registration. Organizations can register with the eRA Commons as they are working through their SAM or Grants.gov registration. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov Applicants must have an active DUNS number and SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from underrepresented racial and ethnic groups as well as individuals with disabilities are always encouraged to apply for FDA support.
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the SF424 (R&R) Application Guide.
This FOA does not require cost sharing as defined in the HHS Grants Policy Statement.
- If an applicant is the recipient of a Food Protection Task Force conference grant with a project period extending beyond September 2018 and the applicant applies for the Food Protection Task Force Funding Option under either Funding Track 1 or Funding Track 2, the pre-existing Food Protection Task Force conference grant will be subject to termination.
Applicant organizations may not submit more than one application.
The FDA will not accept duplicate or highly overlapping applications under review at the same time. This means that the FDA will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
Buttons to access the online ASSIST system or to download application forms are available in Part 1 of this FOA. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
It is critical that applicants follow the instructions in the Research Instructions in the SF424 (R&R) Application Guide, including Supplemental Grant Application Instructions except where instructed in this funding opportunity announcement to do otherwise. Conformance to the requirements in the Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
For information on Application Submission and Receipt, visit Frequently Asked Questions Application Guide, Electronic Submission of Grant Applications.
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows FDA staff to estimate the potential review workload and plan the review.
By the date listed in Part 1. Overview Information, prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed activity
- Name(s), email address(es), and telephone number(s) of the PD(s)/PI(s)
- Names of other key personnel
- Participating institution(s)
- Number and title of this funding opportunity
The letter of intent should be sent via electronic mail as a PDF file with the FOA Number and the Institution's Name in the message subject heading to:
Email: [email protected]
The deadlines to submit a Letter of Intent are: November 17, 2017; September 1, 2018; September 1, 2019; September 1, 2020; September 1, 2021.
A technical session will be held for prospective applicants in December, 2017 (for future years' applications, technical sessions will be held in October of 2018, 2019, 2020 and 2021). The conference call information will be provided to prospective applicants that submit a letter of intent. The technical session will provide an overview of the submission requirements and allow prospective applicants an opportunity to ask questions regarding the application process. Participation in the technical session is optional, but strongly encouraged.
All page limitations described in the SF424 Application Guide and the Table of Page Limits must be followed, with the following exceptions or additional requirements:
- For this specific FOA, the Research Strategy section is limited to 30 pages:
- 10 pages for MFRPS (Development or Maintenance)
- 10 pages for RRT (Development or Maintenance);
- 5 pages for FPTF; and
- 5 pages for Special Project.
The following section supplements the instructions found in the SF424 (R&R) Application Guide and should be used for preparing an application to this FOA.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed with the following additional instructions:
Applications requesting multiple years of support must complete and submit a separate detailed budget breakdown and narrative justification for each year of financial support requested.
- Applicants must project their eligibility and interest in funding options over the five (5) year cooperative agreement, as options to expand participation in funding tracks after the award is made will be limited.
- When a state program transitions from Funding Track 1 to Funding Track 2 during the course of the five (5) year cooperative agreement (e.g., in Year 3), that state program also becomes eligible for the additional funding opportunities available under Funding Track 2 (RRT, Special Projects, etc.).
- Each of the funding tracks selected by the applicant must have a detailed budget justification for each year in which funding is requested.
Where personnel costs are requested, documentation must be provided to clearly associate these costs with the specific goals and deliverables.
For each funding option selected under this cooperative agreement (i.e., MFRPS Development or MFRPS Maintenance, RRT Development or RRT Maintenance, FPTF and Special Project), the applicant must clearly and separately describe all the associated costs in the narrative budget justification.
RRT Development/Maintenance Funding Option:
- Applicants may also apply for personnel, training, and surveillance sample analysis if they have the necessary equipment and it will be available for these projects.
- A portion of budgeted travel funds must be set aside to attend:
- MFRPS Development/Maintenance Funding Option: Key personnel (minimum of 2) to attend an annual face-to-face meeting (as determined by FDA) as a condition of the award.
- MFRPS Development/Maintenance Funding Option: Training needs to support the FDA food inspection contract should be anticipated and budgeted for accordingly.
- RRT Development/Maintenance Funding Option (only applicable if applying for RRT Development or Maintenance funding option): an annual face-to-face meeting of the RRT States and FDA Headquarters and District Offices, as well as the biennial Integrated Foodborne Outbreak Response Management (InFORM) Conference, which is held in odd number years and the Regional PulseNet/OutbreakNet meetings held in non-InFORM years (a minimum of two (2) key personnel for the RRT Annual Meeting and at least one (1) person representing the RRT to InFORM and the Regional PulseNet/OutbreakNet meeting).
- If an applicant is requesting indirect costs as part of their budget, a copy of the most recent Federal indirect cost rate or F&A agreement must be provided as part of the application submission. This agreement should be attached to the RESEARCH & RELATED Other Project Information Component as line #12 'Other Attachments'.
- If the applicant organization has never established an indirect cost rate and/or does not have a negotiated Federal indirect cost rate agreement, a de minimis indirect cost rate of 10 percent (10%) of modified total direct costs (MTDC) will be allowed. MTDC means all direct salaries and wages, applicable fringe benefits, materials and supplies, services, travel, and subaward and subcontracts up to the first $25,000 of each subaward or subcontract. MTDC excludes equipment, capital expenditures, charges for patient care, rental costs, tuition remission, scholarships and fellowships, participant support costs and the portion of each subaward and subcontract in excess of $25,000.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed. All applications must include the following information:
Template cover sheet, including the following table indicating Funding Track(s) and Funding Options selected for each of the five (5) years of the cooperative agreement (downloadable template available at http://www.fda.gov/ForFederalStateandLocalOfficials/FundingOpportunities/GrantsCoopAgrmts/ucm539096.htm). For each year, list the amount requested under each option, and the total award amount for each year.
Year Funding Track 1 Funding Track 2 Total Award
MFRPS Dev. FPTF MFRPS Maint. FPTF RRT Dev. RRT Maint. Spec. Proj.
1
2
3
4
5
- Identify specific personnel responsible for implementation of each funding track selected in the application (e.g., MFRPS, RRT, Food Protection Task Force and Special Project). These personnel may be different from the Principal Investigator (PI) on the overall cooperative agreement.
- For the MFRPS Development or Maintenance funding option, there must be at least one dedicated full time employee/equivalent (FTE) to lead day to day oversight, such as a MFRPS Coordinator.
- Demonstrate the availability of adequately trained staff and the criteria and ability to hire and/or train personnel to meet the deliverables of the cooperative agreement. This should include a description of training needs to support MFRPS Standard 2 and requirements of the FDA food inspection contract (allowable expenses under this cooperative agreement).
- Provide justification for hiring new staff, including qualifications, training needs, and new equipment needs.
- Demonstrate the ability to satisfy the reporting requirements outlined in section VI.3 of this announcement.
- Demonstrate the ability to fully participate in initiatives supporting the cooperative agreement, such as an annual face-to-face meeting (as determined by FDA), committees, conference calls, sharing of best practices, on-site visits, program assessment validation assessments (PAVA), and full program assessments.
- For applicants enrolled in the MFRPS for a minimum of twelve (12) months, a copy of the program self-assessment, strategic improvement plan, and a description of the progress and accomplishments of the program in achieving conformance with the MFRPS must be included in the application. The program self-assessment and strategic improvement plan should be included as appendices following the directions in the SF-424 Guide.
- A Strategic Improvement Plan means a type of improvement plan that includes the following information: (1) the individual element or documentation requirement of the standard that was not met, (2) improvements needed to meet the program element or documentation requirement of the standard, (3) projected completion dates for each task, (4) personnel responsible, and (5) date completed.
- Describe any identified or potential obstacles in achieving and maintaining conformance with the MFRPS and approaches to overcome these obstacles.
- If the applicant is located in a state with a supporting laboratory that receives funding under an active FDA cooperative agreement for maintaining and/or expanding ISO 17025 accreditation for analysis of human food, the applicant must demonstrate the ability to provide for the collection of samples (FDA regulated products only) to support laboratory capacity development and product surveillance. The applicant must also demonstrate the ability to perform any enforcement or other follow-up activities based on sample results. Sampling plans will be developed in cooperation with the laboratory to support the objectives of both programs.
- Outline a detailed methodology to accomplish the work, as described in this announcement.
There must be a separate and distinct methodology section for each funding option included in the application. See page limitations (Section IV of the FOA).
Additional items to be included in MFRPS Development Funding Option (Funding Track 1) Applications
- Demonstrate the ability to develop and implement a comprehensive strategic improvement plan that will result in conformance with the MFRPS, including new versions, within 5 years of receiving cooperative agreement funding to support MFRPS under a previous agreement or this cooperative agreement.
Additional items to be included in MFRPS Maintenance Funding Option (Funding Track 2) Applications
- Demonstrate the ability to implement a comprehensive strategic improvement plan that results in the program maintaining conformance with the MFRPS, including new versions.
- Submit your most recent assessment report provided by the Audit Staff as an appendix following the directions in the SF-424 Guide.
Additional items to be included in RRT Development or Maintenance Funding Option (Funding Track 2) Applications
- Demonstrate the applicant agency's commitment to and support for this cooperative agreement funding track. Also demonstrate commitment from other RRT member agencies external to the applicant agency (at a minimum, entities representing animal food, epidemiology and laboratory) in the form of a letter(s) of support.
For RRT Maintenance funding track:
- Submit most recent RRT Capability Assessment Tool and Activity Table as appendix following the directions in the SF-424 Guide.
- Demonstrate the impact/benefit/effectiveness of the RRT for real-life HAF incidents (outbreaks, natural disasters and other contamination events). Specifically, demonstrate that the RRT consistently: 1) utilizes the RRT in activation (ICS) mode to respond to real-life HAF contamination events; 2) conducts multi-agency/multi-disciplinary investigations, particularly on-site investigations and environmental assessments to document contributing factors and environmental antecedents; 3) documents lessons learned and other investigation findings in After Action Reviews.
- Describe how lessons learned and investigation findings have been cycled back into process improvement or prevention efforts (e.g., training for investigators, updating procedures, outreach/education for industry, publishing investigation findings for academia, industry and other public health partners to learn from).
- Describe steps taken or planned to shorten the time between agency notification of an incident and implementation of effective control measures.
For RRT Development funding track:
- Demonstrate adequate need for advanced capacity and capabilities to respond to all hazards HAF emergencies, as evidenced by: average number of natural disasters affecting HAF firm operations; average number of recalls of HAF commodities resulting from firms in the state or with distribution in the state (by class); average number of HAF outbreaks (especially those involving FDA regulated commodities) where there were illnesses or distribution/processing/production of the contaminated product in the state.
- Describe how the applicant agency plans to work with Federal/State/Local RRT member agencies/partners to develop, share and operationalize capacity/capabilities to rapidly respond to all-hazards HAF emergencies (with the ultimate goal of shortening the time between agency notification and implementation of effective control measures), as well as supporting surveillance and post-response/prevention activities.
Additional items to be included in the Special Project Funding Option (Funding Track 2) Applications
- Indicate whether the proposed project is a MFRPS or RRT-related special project.
- If a MFRPS Special Project, please indicate how the project exceeds the criteria in MFRPS program elements. If a RRT Special Project, please indicate how the project complements (is not redundant) activities proposed under the RRT funding option.
- Describe the plan for completing the project in its entirety during the proposed timeframe.
- If pertinent to the proposed project, describe how relationships with appropriate partner organizations been established.
Resource Sharing Plan: Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the SF424 (R&R) Application Guide, with the following modification:
All applications, regardless of the amount of direct costs requested for any one year, should address a Data Sharing Plan.
Appendix:
Do not use the Appendix to circumvent page limits. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide.
When conducting clinical research, follow all instructions for completing PHS Inclusion Enrollment Report as described in the SF424 (R&R) Application Guide.
All instructions in the SF424 (R&R) Application Guide must be followed.
Foreign (non-U.S.) institutions are not eligible to apply
See Part 1. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
Part I. Overview Information contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday, the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies). Applicants must then complete the submission process by tracking the status of the application in the eRA Commons, FDA's electronic system for grants administration. eRA Commons and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Late applications will not be accepted for this FOA.
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the SF424 (R&R) Application Guide.
This initiative is not subject to intergovernmental review.
All FDA awards are subject to the terms and conditions, cost principles, and other considerations described in the HHS Grants Policy Statement.
Pre-award costs are allowable only as described in the HHS Grants Policy Statement.
Program funds may not be used for any purpose other than those directly tied to the goals of the cooperative agreement.
Non-allowable costs:
- Facilities and work reimbursed under the FDA human food safety inspection contract and other funding mechanisms must remain distinct and separate from the cooperative agreement.
- Vehicle purchases are not permitted.
- Cooperative agreement funds may not be utilized for new building construction; however, remodeling of existing facilities is allowed, provided that remodeling costs do not exceed 10% of the grant award amount.
- Clothing and uniforms with the exception of personal protective equipment (PPE).
- Other items listed in the HHS Grants Policy Statement or Notice of Award.
Additional funding restrictions may be part of the Notice of Award.
Applications must be submitted electronically following the instructions described in the SF424 (R&R) Application Guide. Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit Applying Electronically. If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Guidelines for Applicants Experiencing System Issues. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile Component of the SF424(R&R) Application Package. Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to FDA. See Section III of this FOA for information on registration requirements.
The applicant organization must ensure that the DUNS number it provides on the application is the same number used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the SF424 (R&R) Application Guide.
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the assigned Grants Management Specialist and responsiveness by components of participating organizations, FDA. Applications that are incomplete, non-compliant and/or nonresponsive will not be reviewed.
Applicants are required to follow the instructions for post-submission materials, as described in NOT-OD-13-030.
Reviewers will consider each of the review criteria below in the determination of merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major impact. For example, a project that by its nature is not innovative may be essential to advance a field.
MFRPS DEVELOPMENT AND MAINTENANCE SCORING CRITERIA
1) Effective use of grant funds (10 Points): Is the proposed budget relevant and impactful towards achieving the goals of the cooperative agreement?
2) Significance (25 points): Does the applicant demonstrate the ability to develop or implement a comprehensive approach that results in the program reaching conformance within five (5) years of receiving funding (Development Funding Track 1) or maintaining conformance with the MFRPS (Maintenance Funding Track 2), including new versions? Have potential obstacles to achieving and maintaining conformance been identified, along with approaches to overcome these obstacles? For applicants located in a state with a supporting laboratory that receives funding under an active FDA cooperative agreement for maintaining and/or expanding ISO 17025 accreditation for analysis of human food, does the project outline a sampling plan to support laboratory capacity development and product surveillance?
3) Investigator(s) and Key Personnel (20 points): Does the application provide justification for hiring new staff, including qualifications, training needs, and new equipment needs? Is there at least one dedicated full time employee/equivalent (FTE) to lead day to day oversight of this project, such as a MFRPS Coordinator? Is there evidence of adequate agency management support, as evidenced by the selection of PD/PI(s) for the project?
4) Maintenance (20 points): Does the applicant demonstrate the ability to fully participate in initiatives supporting the cooperative agreement? Does the applicant outline strategies to maintain conformance with the MFRPS ?
5) Approach (25 points): Are the overall strategy, methodology, and analyses well-easoned and appropriate to accomplish the specific goals of the project? Have adequate resources (including staff and infrastructure) been proposed in the application budget to meet the objectives of the cooperative agreement? Is there adequate demonstration of effectiveness in working with federal, state, and local partners and other appropriate organizations to implement the goals of the cooperative agreement?
RRT DEVELOPMENT SCORING CRITERIA
1) Effective use of grant funds (10 Points): Is the proposed budget relevant and impactful towards achieving the goals of the cooperative agreement?
2) Established relationships (40 points): Has the applicant established relationships with partners in other, relevant organizations (e.g., letters of support/commitment from animal food program, laboratory and epidemiology programs; steps taken to date to establish a RRT structure/membership) to collaborate as a multi-jurisdictional, multi-disciplinary RRT to respond to all hazards HAF emergencies?
3) Anticipated impact/benefit/effectiveness of RRT development (50 points): Does the applicant have a plan in place to work with Federal/State/Local RRT member agencies/partners to develop, share and operationalize capacity/capabilities to rapidly respond to all-hazards HAF emergencies (with the ultimate goal of shortening the time between agency notification and implementation of effective control measures), as well as supporting surveillance and post-response/prevention activities? Does the applicant demonstrate adequate need for advanced capacity and capabilities to respond to all hazards HAF emergencies, as evidenced by: average number of natural disasters affecting HAF firm operations; average number of recalls of HAF commodities resulting from firms in the state or with distribution in the state (by class); average number of HAF outbreaks (especially those involving FDA regulated commodities) where there were illnesses or distribution/processing/production of the contaminated product in the state?
RRT MAINTENANCE SCORING CRITERIA
1) Effective use of grant funds (10 Points): Is the proposed budget relevant and impactful towards achieving the goals of the cooperative agreement?
2) Established relationships (40 points): Does the applicant demonstrate mature, strong relationships with Federal/State/Local RRT member agencies/partners and commitment from these member agencies/partners to collaborate as a multi-jurisdictional, multi-disciplinary RRT to respond to all hazards HAF emergencies (e.g., letters of support/commitment, description of current level of engagement and working relationship, etc.)? Particular importance should be stressed on relationships with core RRT member agencies: FDA District Office, State Food Regulatory Programs (all commodity areas), State Feed Regulatory Programs, State Epidemiology Programs and State Laboratory Programs; as well as key auxiliary RRT member agencies: Local Agencies, State Veterinarian’s Office/Board of Animal Health, etc.
3) Track record of impact/benefit/effectiveness of RRT performance (50 points): Does the applicant adequately demonstrate the impact/benefit/effectiveness of the RRT for real-life HAF incidents (outbreaks, natural disasters and other contamination events)? Specifically, does the RRT consistently: 1) utilize the RRT in activation (ICS) and response (non-ICS) mode to respond to real-life HAF contamination events (e.g., number of responses and activations); 2) conduct multi-agency/multi-disciplinary investigations, particularly on-site investigations and environmental assessments to document contributing factors and environmental antecedents (e.g., number of joint investigations); 3) document lessons learned and other investigation findings in After Action Reviews (e.g., number of After Action Reports)? Does the applicant describe how these lessons learned and investigation findings have been cycled back into process improvement or prevention efforts (e.g., training for investigators, updating procedures, outreach/education for industry, publishing investigation findings for academia, industry and other public health partners to learn from)? Does the applicant describe steps taken or planned to shorten the time between agency notification of an incident and implementation of effective control measures?
FPTF SCORING CRITERIA
1) Effective use of grant funds (10 Points): Is the proposed budget relevant and impactful towards achieving the goals of the cooperative agreement?
2) Integration (40 points): Demonstrate the ability to bring together the FPTF stakeholders for a meeting/forum, including: Food Regulatory, Academia, and Industry. Promote the concept of integrated Food Safety System (IFSS), such as: Collaborate on integration projects and share innovative outcomes nationally; Include plans to collaborate with other FPTF's on integration; Work with stakeholders to adopt and implement the appropriate regulations (Food Code, CFR, FSMA etc.); Work on current projects that benefit public health including prevention, intervention, response, and post-response.
3) Replicability (25 Points): Does the applicant plan to share or otherwise make available any developed resources, materials or information to all stakeholders (including other FPTFs) for them to access and use/replicate?
4) Significance (15 Points): Task force mission and objectives are clearly identified and address one of the following: routine communication between FPTF stakeholders, topics of concern to stakeholders, training, improving inspections/investigations, foodborne illness prevention, intervention and/or response, working together to achieve compliance with food regulations/laws.
5) Communication (10 Points): Program addresses how communication and collaboration with key stakeholders (including but not limited to regulatory agencies, academia, industry, consumers, State legislators, Boards of Health and Agriculture, and other interested parties) will support an integrated food safety system.
SPECIAL PROJECTS SCORING CRITERIA (MFRPS and RRT)
1) Effective use of grant funds (10 Points): Is the proposed budget relevant and impactful towards achieving the goals of the cooperative agreement?
2) Significance (25 points): Does the project address support innovation and integration in a nationally Integrated Food Safety System (IFSS) using the MFRPS or RRT framework? If applying for a MFRPS Special Project: does the project exceed the criteria in MFRPS program elements? If applying for a RRT Special Project: does the project complement (does not duplicate) activities proposed under the RRT funding option?
3) Innovation (25 points): Does the project introduce an innovative approach?
4) Impact and ability to replicate across state programs (applicability to others) (20 points): Will project outcomes, data, or lessons learned be shared with FDA and other State programs to improve their capabilities/capacity?
5) Approach (20 points): Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific goals of the project? Can the project be completed within the proposed timeframe? If pertinent to the proposed project, have relationships with appropriate partner organizations been established?
As applicable for the project proposed, reviewers will evaluate the following additional items, but will not give separate scores for these items and should not consider them in providing an overall score.
Budget and Period of Support
The budget and the requested period of support will be reviewed as part of the objective review criteria, above.
Generally not applicable. Reviewers should bring any concerns to the attention of the assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of the assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of the assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of the assigned Grants Management Specialist.
Not Applicable.
Not Applicable.
Not Applicable.
Not Applicable
Generally not applicable. Reviewers should bring any concerns to the attention of the assigned Grants Management Specialist.
Reviewers will comment on whether the following Resource Sharing Plans, or the rationale for not sharing the following types of resources, are reasonable: (1) Data Sharing Plan; (2) Sharing Model Organisms; and (3) Genomic Data Sharing Plan (GDS).
Generally not applicable. Reviewers should bring any concerns to the attention of the assigned Grants Management Specialist.and ensuring the validity of those resources.
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
Applications will be evaluated for scientific and technical merit by an Objective Review Committee using the stated review criteria.
As part of the objective review, all applications:
- Will receive a written critique.
Appeals of objective review will not be accepted for applications submitted in response to this FOA.
Applications will compete for available funds with all other recommended applications submitted in response to this FOA. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by objective review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
Successful applicants will be notified of additional information that may be required or other actions leading to an award. The decision not to award a grant, or to award a grant at a particular funding level, is discretionary and is not subject to appeal to any FDA or HHS official or board.
1. Award Notices
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the grantee’s business official.
Awardees must comply with any funding restrictions described in Section IV.5. Funding Restrictions. Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this FOA will be subject to terms and conditions found in the HHS Grants Policy Statement.
All FDA grant and cooperative agreement awards include the HHS Grants Policy Statement as part of the NoA.
Recipients of federal financial assistance (FFA) from HHS must administer their programs in compliance with federal civil rights law. This means that recipients of HHS funds must ensure equal access to their programs without regard to a person’s race, color, national origin, disability, age and, in some circumstances, sex and religion. This includes ensuring your programs are accessible to persons with limited English proficiency. HHS recognizes that research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), FDA awards will be subject to the Federal Awardee Performance and Integrity Information System (FAPIIS) requirements. FAPIIS requires Federal award making officials to review and consider information about an applicant in the designated integrity and performance system (currently FAPIIS) prior to making an award. An applicant, at its option, may review information in the designated integrity and performance systems accessible through FAPIIS and comment on any information about itself that a Federal agency previously entered and is currently in FAPIIS. The Federal awarding agency will consider any comments by the applicant, in addition to other information in FAPIIS, in making a judgment about the applicant s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 45 CFR Part 75.205 Federal awarding agency review of risk posed by applicants. This provision will apply to all FDA grants and cooperative agreements.
HHS provides general guidance to recipients of FFA on meeting their legal obligation to take reasonable steps to provide meaningful access to their programs by persons with limited English proficiency. Please see https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/index.html. The HHS Office for Civil Rights also provides guidance on complying with civil rights laws enforced by HHS. Please see http://www.hhs.gov/ocr/civilrights/understanding/section1557/index.html; and https://www.hhs.gov/civil-rights/for-providers/laws-regulations-guidance/index.html. Recipients of FFA also have specific legal obligations for serving qualified individuals with disabilities. Please see http://www.hhs.gov/ocr/civilrights/understanding/disability/index.html. Please contact the HHS Office for Civil Rights for more information about obligations and prohibitions under federal civil rights laws at https://www.hhs.gov/ocr/about-us/contact-us/index.html or call 1-800-368-1019 or TDD 1-800-537-7697. Also note it is an HHS Departmental goal to ensure access to quality, culturally competent care, including long-term services and supports, for vulnerable populations. For further guidance on providing culturally and linguistically appropriate services, recipients should review the National Standards for Culturally and Linguistically Appropriate Services in Health and Health Care at http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=2&lvlid=53.
FDA considers the sharing of research resources developed through FDA-sponsored research an important means to enhance the value and further the advancement of research. When research resources have been developed with FDA funds and the associated research findings published, those findings must be made readily available to the scientific community.
Upon acceptance for publication, scientific researchers must submit the author’s final manuscript of the peer-reviewed scientific publication resulting from research supported in whole or in part with FDA funds to the NIH National Library of Medicine's (NLM) PubMed Central (PMC). FDA defines the author's final manuscript as the final version accepted for journal publication, which includes all modifications from the publishing peer review process. The PMC archive is the designated repository for these manuscripts for use by the public, health care providers, educators, scientists, and FDA. Please see the FDA Public Access Policy.
Additional terms and conditions regarding FDA regulatory and FDA Office of Regulatory Affairs programmatic requirements may be part of the Notice of Award.
Cooperative Agreement Terms and Conditions of Award
The administrative and funding instrument used for this program is the cooperative agreement, an assistance instrument (rather than an acquisition instrument), in which substantial FDA programmatic involvement with the awardees is anticipated during the performance of the activities. Under the cooperative agreement, FDA’s objective is to support and stimulate the recipient’s activities by involvement in and otherwise working jointly with the award recipient in a partnership role; it is not to assume direction, prime responsibility, or a dominant role of activities. Consistent with this concept, the dominant role and prime responsibility resides with the awardee for the project as a whole, although specific tasks and activities may be shared between the awardee and the FDA as defined below.
a) Cooperative Agreement--Project Director/Principal Investigator Rights and Responsibilities:
The Project Director/Principal Investigator (PD/PI) retains the primary responsibility and dominant role for planning, directing, and executing the proposed project, with FDA/ORA staff being substantially involved as a partner with the PD/PI, as described below.
The PD/PI will maintain general oversight for ensuring compliance with the financial and administrative aspects of the award, as well as ensuring that all staff have sufficient clearance and/or background checks to work on this project. This individual will work closely with designated officials within the recipient organization and with partner organizations to create and maintain necessary documentation, including both technical and administrative reports; prepare justifications; appropriately acknowledge Federal support in publications, announcements, news programs, and other media; and ensure compliance with other Federal, regulatory, and organizational requirements.
b) Cooperative Agreement--FDA Responsibilities:
An FDA Project Officer (PO) will be assigned and named in the NoA. The FDA PO is the official responsible for the programmatic, scientific, and/or technical aspects of assigned applications and cooperative agreements. The FDA PO will have substantial programmatic involvement that is above and beyond the normal stewardship role in awards as described below.
The PO will have substantial involvement in the design, implementation, and evaluation of program activities, and dissemination of program results and outcomes, above and beyond routine grant monitoring. Substantial involvement by FDA/ORA includes, but is not limited to, the following:
- Provide guidance, direction, and technical assistance in project planning, implementation, and evaluation;
- Provide subject matter expertise, programmatic assistance, and evaluation services to support program studies and activities;
- Actively monitor the supported program via telephone conversations, webinars, e-mails, written correspondence, or periodic site visits;
- Evaluate the supported program, including development of program-level performance measures, consistent data collection, and reporting procedures and protocols;
- Convene trainings, meetings, conference calls, and site visits with grantee to facilitate collaboration and information sharing;
- Participate in data analysis, interpretation of findings, and where appropriate, co-authorship of publications;
- Development of programs to meet the FDA mission;
- Provision of programmatic technical assistance;
- Post-award monitoring of project/program performance, including review of progress reports and making site visits; and other activities complementary to those of the FDA.
Unless another governance structure is mutually agreed upon, the PO will serve as the primary point of contact for the dissemination of FDA policy and milestones/objectives work planning.
Monitoring Activities
Periodic program monitoring will be conducted by FDA on an ongoing basis which may include telephone conversations between the Principal Investigator and the Project Officer/Grants Management Officer/Grants Management Specialist, site visits and the review of written reports.
The Annual Progress Report will be due as part of the Research Performance Progress Report (RPPR) and is due no later than 60 days prior to the start date of the next budget period start date.
Grants with Multiple Years: In order to receive future funding, the grantee is required to submit the Research Performance Progress Report (RPPR). The Annual Progress Report will be due as part of the Research Performance Progress Report (RPPR) and is due no later than 60 days prior to the start date of the next budget period start date. This report should cover all activities/work that took place during the current budget performance period noted in your Notice of Grant Award (NGA).
Financial Reporting:
A. Cash Transaction Reports
The Federal Financial Report (FFR) has a dedicated section to report Federal cash receipts and disbursements. For recipients this information must be submitted quarterly directly to the Payment Management System (PMS) using the web-based tool. Quarterly reports are due 30 days following the end of each calendar quarter. The reporting period for this report continues to be based on the calendar quarter. Questions concerning the requirements for this quarterly financial report should be directed to the PMS.
B. Financial Expenditure Reports
A required Federal Financial Report (FFR) must be submitted annually. FDA now requires all annual financial expenditure reports to be submitted electronically using the Federal Financial Report (FFR) system located in the eRA Commons. This includes all initial FFRs being prepared for submission and any revised FSR/FFRs being submitted or re-submitted to FDA. Paper expenditure/FFR reports will not accepted.
Annual FFRs must be submitted for each budget period no later than 90 days after the end of the calendar quarter in which the budget period ended. The reporting period for an annual FFR will be that of the budget period for the particular grant; however, the actual submission date is based on the calendar quarter. Failure to submit timely reports may affect future funding.
4. Closeout Requirements (when applicable): A Final Program Progress Activity Report, Final Federal Financial Report SF-425, Final Invention Statement HHS-568 (if applicable), Tangible Personal Property Report SF-428, and Statement of Disposition of Equipment (if applicable) must be submitted within 90 days after the expiration date of the project period.
5. A non-Federal entity that expends $750,000 or more during the non-Federal entity's fiscal year in Federal awards must have a single or program-specific audit conducted for that year in accordance with the provisions of 45 CFR 75, Subpart F-Audit Requirements. Audits must be completed and submitted electronically to the Federal Audit Clearinghouse (FAC) within 30 days after receipt of the auditor's report(s), or 9 months after the end of the audit period, i.e., the end of the organization's fiscal year, whichever is earlier. If you need information on your organization’s obligations, please visit the following website: http://harvester.census.gov/sac/. Valuable information is included under the Frequently Asked Questions section of that website.
The grantee organization must comply with all special terms and conditions of the cooperative agreement. Future funding will be dependent on recommendations from the Project Officer. The scope of the recommendation will confirm an acceptable level of performance and continued compliance with all FDA regulatory requirements and conditions of the award. Specific project milestones, reporting requirements, and other project deliverables may be included as a condition of your award. If FDA determines that the state is unable to make adequate progress, FDA may place them in special condition status and may require a corrective action plan.
Grantees developing their MFRPS program will achieve and maintain conformance with the MFRPS (most recent version) within five years. Grantees who have already developed their MFRPS program will maintain conformance by demonstrating implementation of a strategic improvement plan when non-conformance is identified by the State program or FDA.
The grantee must maintain a food safety inspection contract in satisfactory standing with the FDA throughout the cooperative agreement.
If a recipient of multiple FDA awards (cooperative agreements, grants, contracts), the State must be able to account separately for fund expenditures, including employee salaries, wages, and benefits, under those funding mechanisms and this cooperative agreement.
Additional Terms and Conditions for MFRPS Funding track (as applicable)
- Key personnel (minimum of two) must attend an annual face-to-face meeting (as determined by FDA OP) as a condition of the award.
- State manufactured food regulatory programs in the MFRPS Development funding track are expected to achieve conformance with the MFRPS before Year 5 of the cooperative agreement.
- State manufactured food regulatory programs in the MFRPS Maintenance funding track are expected to maintain conformance with the MFRPS throughout the duration of the award.
Additional Terms and Conditions for RRT funding track (as applicable)
- A minimum of two (2) key RRT personnel must attend an annual face-to -face RRT meeting (as determined by FDA OP) and at least one person representing the RRT must attend the biennial Integrated Foodborne Outbreak Response Management (InFORM) Conference (held in odd number years) and the Regional PulseNet/OutbreakNet meetings (held in non-InFORM years) as a condition of the award.
Additional Terms and Conditions for FPTF funding track (as applicable)
- All conference material (promotional materials, agenda, publications and internet sites) related to this project must include an acknowledgement of FDA grant support and a disclaimer stating the following: Funding for this conference was made possible [in part] by [insert grant number] from [insert FDA name]. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Additional Terms and Conditions for Special Project funding track (as applicable)
- FDA reserves a royalty-free, nonexclusive, and irrevocable right to reproduce, publish, or otherwise use for federal purposes any copyrighted works that are outcomes from these funding tracks, including curriculum, course content, objectives, learning outcomes, presentations, manuals, scripts, exercises, handouts, reports, documents or other tangible materials produced by the awardee . FDA may authorize others to reproduce, publish, or otherwise use such works for Federal purposes.
When multiple years are involved, awardees will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the Notice of Award.
For all years of the cooperative agreement, mid-year reports and an end of year program progress report are required. For continuation years, the RPPR will be considered the end of year progress report. Reporting requirements may be adjusted for individual grantees in the Notice of Award.
Mid-year and end of year progress reports must contain the elements below:
- Detailed progress report on the grantee meeting the project milestones detailed in the cooperative agreement, proposal, strategic plan, conditions of the award, etc. Goals and objectives should be outlined in detail and specific progress reported.
- Status report on the hiring and training of cooperative agreement funded personnel and other manufactured food program personnel.
- Status report on the purchasing, development, and operational readiness of any equipment, computers, or software purchased.
- Identify any pending issues or concerns that may affect accomplishing the objectives and goals of the cooperative agreement.
- A corrective action plan must be submitted if the objectives and goals of the cooperative agreement are not being met. The corrective action plan must detail the tasks, responsible personnel, and updated timeframes to ensure satisfactory performance and meet the deliverables required under the grant.
- Summary of grant expenditures and obligations during the current budget period.
Additional requirements for the mid-year progress report:
- Submission of the following documents in the most current version of the MFRPS reviewed and updated within the current budget period (see below list). State programs enrolling in MFRPS for the first time under this cooperative agreement do not need to submit these appendices until the mid-year progress report of Year 2 of the cooperative agreement. These documents may be found in the 2016 version of the MFRPS available at https://www.fda.gov/downloads/ForFederalStateandLocalOfficials/ProgramsInitiatives/RegulatoryPrgmStnds/UCM523944.pdf.
- Appendix 1 or alternate form that is equivalent
- Appendix 2.1 or alternate form that is equivalent
- Appendix 3.1 or alternate form that is equivalent
- Appendices 4.1 or alternate form that is equivalent
- Appendix 5.1 or alternate form that is equivalent
- Appendices 6.1 or alternate form that is equivalent
- Appendix 7.1 or alternate form that is equivalent
- Appendices 8.1 or alternate form that is equivalent
- Appendix 9.1 or alternate form that is equivalent
- Appendix 10.1 or alternate form that is equivalent
- Submission of a strategic improvement plan, as defined in the current version of the MFRPS, updated within the current budget period to demonstrate program advancement in achieving conformance with the MFRPS.
- Additional requirements for the end of year progress report:
- Summary of grant expenditures and obligations during the current budget period and those anticipated to occur during the budget period. The grantee is required to indicate if a carryover request will be submitted for any remaining funds in the current budget period and the anticipated use of the funds in the upcoming budget period. The grantee is strongly encouraged to submit the carryover request with the continuation application.
- For each funding track and option selected under this cooperative agreement (i.e., MFRPS Development or MFRPS Maintenance, RRT Development or RRT Maintenance, FPTF and Special Project), the grantee must clearly and separately describe all the associated costs in the narrative budget justification.
- The grantee must provide an estimate (in total dollars) of in-kind contributions toward accomplishing the goals of the cooperative agreement during the reporting period. A separate estimate should be provided for each funding option selected under this cooperative agreement (i.e., MFRPS Development or MFRPS Maintenance, RRT Development or RRT Maintenance, FPTF and Special Project).
Special consideration for programs in RRT Maintenance & Development
- All progress reports (mid-year, end of year and final) must contain, but are not limited to the following:
- Progress & achievements for each yearly goal.
- Progress & achievements for other projects, identified by the grantee in the application or subsequent to receiving funding.
- Summary of significant RRT responses or other activities within the timeframe for the report, including status of AAR & lessons learned/recommendations for improvement
- Point of Contact and Project Key Personnel
- Pending Issues/Concerns and Proposed Solutions
Special consideration for programs in FPTF
- The mid-year progress report submitted for the overall cooperative agreement (MFRPS Development or Maintenance Tracks) does not need to include a mid-year progress report for the FPTF funding option. The FPTF funding option must be included in the end of year and final progress reports.
- FPTF progress reports must address the following:
- Provide the number of meetings, trainings, and workshops supported with copies of agendas and supporting materials (handouts, slides, etc.)
- Provide a copy of the meeting, training, workshop etc. sign-in sheet that captures the names, emails, and affiliations of the attendees.
- Discussions and decisions resulting from these activities (reports, recommendations, questions etc.).
- Identify an integration activity to address each year (see FOA Part 2, Section I, Sub-section 3 (FPTF), objective 3, above) and provide an update on the activity in the annual report.
- Identify if there were issues encountered during the implementation and/or adoption of FSMA or other rules/codes/ordinances.
- Provide a detailed outline of funding in the budget (based on allowable expenses categories).
Special consideration for programs in Special Projects:
- Mid-year and end of year progress reports for the Special Projects funding option should describe progress made to date.
- The final progress report (after the conclusion of the entire cooperative agreement) must include an evaluation/final report, including: lessons learned, results, and analysis of effectiveness and impact.
Final progress report: additional requirements (applies only after the conclusion of the entire cooperative agreement):
- Full written documentation of the project and summaries of accomplishments and goals, as described in the grant application. The documentation must be in a form and contain sufficient detail such that other State, local, and tribal governments could reproduce the final project.
- The most recent assessment by FDA should verify the program is maintaining conformance with the MFRPS.
The Federal Funding Accountability and Transparency Act of 2006 (Transparency Act), includes a requirement for awardees of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All awardees of applicable FDA grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over $25,000.
At the conclusion of the multi-year cooperative agreement, a final progress report, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the HHS Grants Policy Statement, to be submitted within ninety (90) days of the expiration date as noted on the Notice of Grant Award. Alternatively, a final Progress Report is due when an award is relinquished, when a recipient changes institutions or when an award is terminated.
In accordance with the regulatory requirements provided at 45 CFR 75.113 and Appendix XII to 45 CFR Part 75, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (currently the Federal Awardee Performance and Integrity Information System (FAPIIS)). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 45 CFR Part 75 Award Term and Conditions for Recipient Integrity and Performance Matters.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the HHS Grants Policy Statement.
The Federal Funding Accountability and Transparency Act of 2006 (Transparency Act), includes a requirement for awardees of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All awardees of applicable FDA grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over $25,000.
In accordance with the regulatory requirements provided at 45 CFR 75.113 and Appendix XII to 45 CFR Part 75, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (currently FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 45 CFR Part 75 Award Term and Conditions for Recipient Integrity and Performance Matters.
We encourage inquiries concerning this funding opportunity
and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons
registration, submitting and tracking an application, documenting system
problems that threaten submission by the due date, post submission issues)
Finding Help Online: http://grants.nih.gov/support/ (preferred method of contact)
Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
Grants.gov
Customer Support (Questions
regarding Grants.gov registration and submission, downloading forms and
application packages)
Contact Center Telephone: 800-518-4726
Web ticketing system: https://grants-portal.psc.gov/ContactUs.aspx
Email: [email protected]
Wendy Campbell
Office of Regulatory Affairs (ORA)
Telephone: 615-310-0483
Email: [email protected]
Brett Weed
Office of Regulatory Affairs (ORA)
Telephone: 404-253-2268
Email: [email protected]
Daniel Lukash
Office of Acquisitions & Grants Services (OAGS)
Food and Drug Administration
Telephone: 240-402-7596
Email: [email protected]
Daniel Lukash
Office of Acquisitions & Grants Services (OAGS)
Food and Drug Administration
Telephone: 240-402-7596
Email: [email protected]
All awards are subject to the terms and conditions, cost principles, and other considerations described in the HHS Grants Policy Statement .
Awards are made under the authorization of Section 317R of the Public Health Service Act (42 USC 247b-20), and Section 1004 of the Food and Drug Administration Amendments Act (21 USC 2104).

