Notice Number: NOT-MH-10-007
Key Dates
Release Date: December 9, 2009
Receipt Date: January 19, 2010
Earliest Anticipated Start Date: April 2, 2010
Issued by
National Institute of Mental Health (NIMH), (http://www.nimh.nih.gov/)
National Institute of Neurological Disorders and Stroke (NINDS) (http://www.ninds.nih.gov/)
National Institute on Drug Abuse (NIDA) (http://www.nida.nih.gov/)
Purpose
The National Institute of Mental Health (NIMH), National Institute of Neurological Diseases and Stroke (NINDS), and National Institute on Drug Abuse (NIDA) announce the availability of funds to support a single administrative supplement for an NIH grantee, currently funded for human brain banking, to extend brain collection efforts to include receipt, processing, and storage of adult human normal control postmortem brains and spinal cords from the NIH Roadmap Genotype-Tissue Expression (GTEx) Project. The proposed effort must be within the scope of the peer-reviewed activities specified within the parent grant. The length of the supplement will be limited to the duration of the parent grant, up to five years. This is a one-time solicitation.
Background
The Genotype-Tissue Expression (GTEx) project, an NIH Roadmap Initiative (http://nihroadmap.nih.gov/GTEx), aims to provide a resource to the scientific community with which to study the relationship between genetic variation and regulation of gene expression. This project will collect and analyze multiple human tissues from donors who have been characterized for germline genetic variation through dense genotyping. By treating global RNA expression levels as quantitative traits, loci with polymorphisms that are highly correlated with variations in expression will be identified as expression quantitative trait loci, or eQTLs.
The GTEx Pilot Project comprises two main initiatives: (1) Biospecimen Acquisition, and (2) The Laboratory, Data Analysis and Coordinating Center (LDACC), with the latter established through a separate contract mechanism by NHLBI for GTEx (RFP NHLBI-HG-10-01. Biospecimen Acquisition will be organized under the National Cancer Institute’s (NCI) cancer Human Biobank (caHUB) initiative. A diagrammatic view of the functional areas for the GTEx project is shown in Figure 1. Tissue Source Sites (TSS) will be contracted to recruit donors and collect biospecimens.
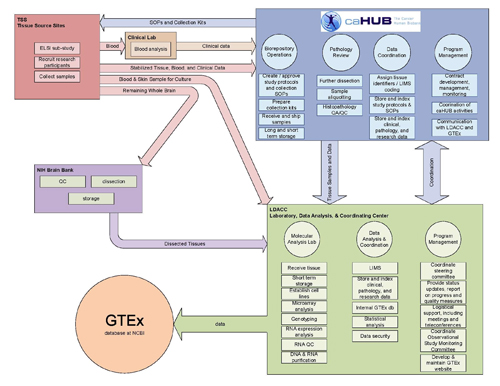
Figure 1. Schematic showing the functional organization of the GTEx pilot program
The GTEx project will begin with a 2.5-year pilot in FY10. As developed at a June 2008 GTEx Workshop (http://nihroadmap.nih.gov/GTEx/workshop0608), the primary goal of the pilot is to assess the feasibility of enrolling 160 donors identified through low post-mortem-interval (PMI) autopsy or organ transplant settings and collecting high-quality RNA from multiple tissues per donor. The precise number of tissues that yield high-quality RNA that can be collected from each donor is not known. The aim of this project is to collect as many different tissue types as is practical, ideally 50 to 70, and to analyze gene expression in at least 50 tissues per donor.
For this Notice, NIMH, NINDS, and NIDA intend to capitalize on the GTEx effort by receiving, storing, and dissecting the postmortem human brains and spinal cords collected from GTEx donors for the GTEx pilot phase and future studies. The purpose of this initiative is to supplement an existing CNS tissue storage facility to store and distribute the brain and spinal cords collected by the GTEx project. Donors will be recruited and tissue will be collected by the GTEx tissue source sites and shipped to the successful applicant for further processing. The successful applicant will be expected to: provide the GTEx TSS with a standardized protocol for CNS tissue removal and stabilization for shipment; perform basic neuropathology quality control on tissue collected shortly after receipt; dissect defined brain areas and ship these to the GTEx Laboratory, Data Analysis and Coordinating Center (LDACC) using provided, standardized containers and tracking procedures, within a defined time period; store remaining brain and spinal cord tissue and associated clinical and other phenotype data using a LIM system to allow tracking and repeated sample dissection from the same donor brain; and provide requested tissues to qualified investigators.
The GTEx project is in a pilot stage for 2010-2011. Depending on the success of the pilot and available funds, the GTEx project may scale up in subsequent years to collect up to 800 additional postmortem tissue donors. Applicants should address their ability to scale up brain storage and sample handling in the event that the full GTEx project is approved and funded.
Eligibility
Before submitting an application for an administrative supplement, grantees are strongly encouraged to communicate with the Program Officer(s) of their funded NIH grant to discuss the planned application.
This program will support one administrative supplement to an existing R01 or R24 grant. The parent grant must have at least one year of active funding remaining at the time of award in order to be eligible. Post-mortem brain processing must be included in the scope of the parent grant. Only one supplement request may be submitted per grant.
Post-Award Requirements
The awardee will be required to participate in GTEx Steering Committee teleconferences, as well as one in-person meeting. A two-page progress report addressing the progress of the supplement-funded activities will be required annually. It should be included as an addition to the progress report of the parent grant or included in the final progress report as a part of the grant close-out process if there is no non-competing application.
Submitting an Application
Applications must be received on or before January 19, 2010. Do not send applications to the Center for Scientific Review. Applicants are encouraged to submit one electronic copy as an e-mail attachment in PDF format and one hard copy (with original signatures of the PI and institutional official) of the application to:
Tiffany Yates
Division of Neuroscience and Basic Behavioral Science
National Institute of Mental Health
6001 Executive Boulevard, Room 7194, MSC 9641
Bethesda, MD 20892-9641
Rockville, MD 20852 (express/courier service)
Email: [email protected]
Requests for an administrative supplement under this program must be submitted on the PHS 398 (rev. 11/2007), available at: http://grants.nih.gov/grants/funding/phs398_ver1107/phs398.html) and must include the following:
1. Cover letter requesting the supplement, identifying this notice, the number and title of the parent grant, and providing full contact information for the PI. A separate title for the supplement activity should also be provided in this letter.
2. Face page.
The title of the project (Box 1) should be the title of the parent grant.
This Notice (number and title) should be cited in Box 2, and the yes box should be checked.
The Principal Investigator (PI) must be the same as the PI on the parent grant.
The remaining items on the face page should be filled out according to the PHS 398 application instructions.
3. Form page 2 (Description, Performance Sites, Key Personnel, Other Significant Contributors, and Human Embryonic Stem Cells) from PHS 398. The project description is that of the administrative supplement, not the parent grant. Any new co-investigator(s) or collaborator(s) should be noted under performance sites, along with their institution(s).
4. Biosketch(es) for any new key co-investigator(s) or collaborator(s), which are not listed within the parent grant, should be provided.. Letters of commitment from these new participants should be included in section 16 (Consultants) of the Research Plan.
5. Resources page(s) for new key personnel not named in the parent grant(s).
6. Proposed budget for the supplement with a budget justification that details the items requested. See Budget Information section below.
7. Research Plan for the supplement, items 2-5 not to exceed five pages. Font size restrictions apply as designated within the PHS398 instructions.
This section should include a description of the aims of the parent grant and the supplement's specific aims (2), background and significance (3), preliminary studies (4), and research design and methods (5). The relationship of the proposed studies to the parent grant should be included under background and significance in section 3.
Items 13 (Select Agent Research), 7 (Bibliography and References Cited), 15 (Consortium/Contractual Arrangements), 17 (Resource Sharing) and 16 (Consultants) should be completed as described in the PHS 398 Instructions.
Item 14 Multiple PDs/PI Leadership Plan is not required.
Appendices and supplemental material will not be accepted.
Review and Award Criteria
Applications that are complete and responsive to the goals of the supplement announcement will be evaluated for scientific and technical merit by a committee of NIH staff. Incomplete applications will not be reviewed. Awards will be determined on the basis of technical feasibility, the experience of the applicant in managing post-mortem tissue, sophistication of the pathology core staff and the track record of the applicant in managing and distributing post-mortem tissue. Preference will be given to supplement requests where the likelihood for success is highest. The added value of the activity to the parent grant(s) will also be taken into consideration. All funding decisions are final and not subject to appeal. Resubmission applications will not be considered.
Budget Information
Applicants may request up to $250,000 in direct costs for the duration of the administrative supplement under this program. The Principal Investigator on the parent NIH grant must use the supplemental funds to support work within the scope of the parent grant to include the collected GTEx CNS tissue. The duration of the award is limited to the duration of the parent grant, not to exceed five years. Facilities and Administrative (F&A) costs will be paid at the full, negotiated rate. Applicants should provide a detailed budget justification for personnel costs, equipment, supplies, and other expenses. The purchase of equipment is allowed. In fiscal year 2010, the participating Institutes plan to commit approximately $400,000 total costs towards this administrative supplement program, with 1 award anticipated.
Inquiries
Applicants are strongly encouraged to discuss their plans for responding to this Notice by phone or e-mail. Scientific inquiries should be directed to the NIH Program Officer who oversees the parent grant associated with the administrative supplement request. General inquiries about the supplement program can be directed to:
Susan Koester, Ph.D.
Division of Neuroscience and Basic Behavioral Science
National Institute of Mental Health
6001 Executive Boulevard, Room 7205, MSC 9645
Bethesda, MD 20892-9645
Telephone: (301) 443-3563
Email: [email protected]

