Department
of Health and Human Services
Issuing Organization
National Institutes of Health
(NIH), (http://www.nih.gov)
Participating Organizations
National Institutes of Health
(NIH), (http://www.nih.gov)
Components of
Participating Organizations
National Institutes of Health
(NIH), (http://www.nih.gov)
This FOA was
developed as an NIH Roadmap initiative.� All NIH Institutes
and Centers (ICs) participate in NIH Roadmap initiatives and activities. The FOA will be
administered by the National Institute on Drug Abuse (NIDA) on behalf of the
NIH.
Title: Facilitating
Interdisciplinary Research via Methodological and Technological
Innovation in the Behavioral and Social Sciences (R21)
Announcement Type
New
NOTICE: Applications submitted in response to this Funding Opportunity Announcement (FOA) for Federal assistance must be submitted electronically through Grants.gov (http://www.grants.gov) using the SF424 Research and Related (R&R) forms and the SF424 (R&R) Application Guide.
APPLICATIONS MAY NOT BE SUBMITTED IN PAPER FORMAT.
This FOA must be read in conjunction with the application guidelines included with this announcement in Grants.gov/Apply for Grants (hereafter called Grants.gov/Apply).
A registration process is necessary before submission and applicants are highly encouraged to start the process at least four weeks prior to the grant submission date. See Section IV.
Request For Applications (RFA) Number: RFA-RM-07-004
Catalog of Federal
Domestic Assistance Number(s)
93.310 �
Key Dates
Release/Posted Date: October 25, 2006
Opening Date: January 23, 2007 (Earliest date an application may be
submitted to Grants.gov)
Letters of Intent Receipt Date(s): January 23, 2007
NOTE: On time submission requires that applications be successfully
submitted to Grants.gov no later than 5:00 p.m. local time (of the applicant
institution/organization).�
Application Submission/Receipt
Date(s): �February 23, 2007 �
AIDS Application Submission/Receipt Date(s): �Not
applicable.
Peer Review Date(s): June 2007�
Council Review Date(s): August 2007
Earliest Anticipated Start Date(s): September 15, 2007
Additional Information To
Be Available Date (Activation Date): Not Applicable
Expiration Date: February 24, 2007
Technical Assistance Conference
Call: November 21, 2006
Frequently
Asked Questions website posted: December 4, 2006
Due Dates for E.O. 12372
�
Not
Applicable
Additional
Overview Content
Executive Summary
- Purpose. This Funding Opportunity Announcement (FOA) solicits applications to develop new/innovative measures, methods, and technologies that support the interdisciplinary integration of human social and/or behavioral science with other disciplines across varying levels of analysis.
- This FOA is part of the NIH Roadmap for Medical Research Initiative.� For more information about this Initiative, please visit: http://nihroadmap.nih.gov/.
- Mechanism of Support. This Funding Opportunity Announcement (FOA) will utilize a modified Exploratory/Developmental (R21) (see Budget and Project Period below).
- Funds Available and Anticipated Number of Awards. $3 million dollars in FY 2007 funds have been committed to fund applications received in response to this FOA.�
- Budget and Project Period. This FOA uses a modified R21 funding mechanism. The total project period for an application submitted in response to this funding opportunity may not exceed four years.� Total costs are limited to $1,200,000 over a four-year period, with no more than $300,000 in total costs allowed in any single year. �
- Approximately 10 awards will be made. Awards issued under this FOA are contingent on the availability of funds and the submission of a sufficient number of meritorious applications.
- R21 grants are not renewable.� Continuation of projects developed under this FOA will be through the regular research project grant mechanism (e.g., R01).� At this time, there are no plans to reissue this FOA.
- Eligible Institutions/Organizations. Public/State Controlled Institution of Higher Education; Private Institution of Higher Education; Nonprofit with 501(c)(3) IRS Status (Other than Institution of Higher Education); Nonprofit without 501(c)(3) IRS Status (Other than Institution of Higher Education); Small Business; For-Profit Organization (Other than Small Business); State Government; U.S. Territory or Possession; Indian/Native American Tribal Government (Federally Recognized); Indian/Native American Tribal Government (Other than Federally Recognized); Indian/Native American Tribally Designated Organization; Non-domestic (non-U.S.) Entity (Foreign Organization); Hispanic-serving Institution; Historically Black Colleges and Universities (HBCUs); Tribally Controlled Colleges and Universities (TCCUs); Alaska Native and Native Hawaiian Serving Institutions; Regional Organization; Eligible agencies of the Federal government; Faith-based or community based organizations.)
- Eligible Project Directors/Principal Investigators (PDs/PIs): Individuals with the skills, knowledge, and resources necessary to carry out the proposed research are invited to work with their institution to develop an application for support. Individuals from underrepresented racial and ethnic groups as well as individuals with disabilities are always encouraged to apply for NIH support.
- Applications must involve interdisciplinary research teams.� Applications which do not propose a research team will be deemed unresponsive.� These interdisciplinary teams must include behavioral and/or social scientists in addition to scientists from other discipline(s).
- Applications accepted under this FOA will be permitted to have more than one PI under the Multiple Principal Investigator Policy.
- Number of Applications. Applicants may submit more than one application, provided each application is scientifically distinct. �
- Application Materials. See Section IV.1 for application materials.
- General
Information. For general
information on SF424 (R&R) Application and Electronic Submission, see
these Web sites:
- SF424 (R&R) Application and Electronic Submission Information: https://grants.nih.gov/grants/funding/424/index.htm
- General information on Electronic Submission of Grant Applications: http://era.nih.gov/ElectronicReceipt/
- Hearing Impaired. Telecommunications for the hearing impaired is available at: TTY 301-451-0088.
- Initial merit review convened by the NIDA Office of Extramural Affairs.
Table of Contents
Part I Overview Information
Part II Full Text of Announcement
Section I. Funding Opportunity
Description
1. Research Objectives
Section II. Award Information
1. Mechanism of Support
2. Funds Available
Section III. Eligibility
Information
1. Eligible Applicants
A. Eligible Institutions
B. Eligible Individuals
2. Cost Sharing or Matching
3. Other - Special Eligibility Criteria
Section IV. Application and
Submission Information
1. Request Application Information
2. Content and Form of Application Submission
3. Submission Dates and Times
A. Submission, Review, and
Anticipated Start Dates
1.
Letter of Intent
B. Submitting an Application Electronically
to the NIH
C. Application Processing
4. Intergovernmental Review
5. Funding Restrictions
6. Other Submission Requirements
Section V. Application Review
Information
1. Criteria
2. Review and Selection Process
A. Additional Review Criteria
B. Additional Review Considerations
C. Sharing Research Data
D. Sharing Research Resources
3. Anticipated Announcement and Award Dates
Section VI. Award Administration
Information
1. Award Notices
2. Administrative and National Policy Requirements
A. Cooperative Agreement Terms and Conditions of Award
3. Reporting
Section VII. Agency Contact(s)
1. Scientific/Research Contact(s)
2. Peer Review Contact(s)
3. Financial/Grants Management Contact(s)
Section VIII. Other Information
- Required Federal Citations
Part II
- Full Text of Announcement
Section I. Funding Opportunity Description
1.
Research Objectives
Purpose
The purpose of this funding opportunity is to advance the understanding of health through development of new/innovative methodologies and technologies to support the interdisciplinary integration of social and/or behavioral scientific disciplines with other disciplines.� This announcement supports proposals that integrate across various levels of analysis, ranging from sub-individual to population levels.� Note that in this FOA any reference to methodologies or methods includes analytic approaches, research design, and measures.�
BackgroundInterdisciplinary, Multi-level Approaches
Behavioral and social sciences have offered significant, fundamental insights into the comprehensive understanding of human health, including knowledge of disease etiology, prevention, and treatment, and of factors critical to the promotion of health and well-being.� Merging scientific insights and technologies gleaned from behavioral and social sciences with approaches from other scientific disciplines offers the promise of further advancing the public health mission of the NIH.�
Distinct disciplinary perspectives contribute significant sources of strength to the overall research enterprise because each discipline has its own intellectual history, experimental and analytic approaches, and theoretical context that produce a unique way of thinking about a problem.� Nevertheless, as scientific capabilities move forward, increasingly sophisticated questions arise, and these often require the convergence of perspectives from multiple disciplines.� Over the years, the Institutes and Centers (ICs) of the National Institutes of Health (NIH) have developed many initiatives, mechanisms and programs to support research falling within a single discipline, as well as multidisciplinary research, wherein different disciplines come together to focus on a circumscribed problem, although the contributions and conceptual framework from the respective disciplines remain quite distinct.
In some cases, however, it is becoming apparent that multidisciplinary research is not sufficient to address, in a comprehensive and effective way, challenging and complex problems in biomedical and behavioral research (such as obesity, diabetes, HIV/AIDS, substance abuse, mental illness, etc.).� Rather, these more complex problems may require interdisciplinary research.� Like multidisciplinary research, interdisciplinary research brings together different disciplines to address a particular issue.� But unlike a multidisciplinary approach, interdisciplinary research integrates concepts and techniques from the contributing disciplines in ways that produce new conceptual frameworks.� Integrating different disciplines in this way holds the promise of opening up currently-unimagined avenues of scientific inquiry, and in the process, may spawn and foster wholly new disciplines.� Historical examples of the evolution of such new disciplines include genomics, which integrates genetics, molecular biology, analytical chemistry, and informatics.� Another example in which multiple disciplines have, in a less directed way, blended and evolved into a new discipline is neuroscience.� Thirty years ago, students of the nervous system might have identified themselves as anatomists, physiologists, or psychologists, but today most would consider themselves neuroscientists.� Importantly, interdisciplinary research does not merely result in new technical approaches, but also new intellectual approaches to conceptualize and think about a research problem.
Although behavioral and social sciences have contributed greatly to the understanding, prevention, and treatment of many of today�s pressing health problems, there is growing recognition that with improved methodologies, behavioral and social sciences could do more to address the complexity of these problems. This complexity results from the interaction of multiple factors related to a health problem, and in many cases is further compounded as the relationships between these components change over time. This complex and dynamic conceptualization is captured in Figure 1 (adapted from Glass & McAtee 2006). Thus, many if not most public health problems can be viewed as occurring within a context of nested hierarchies, from micro to macro levels (�from cells to society�), relationships among which change over time and across development.
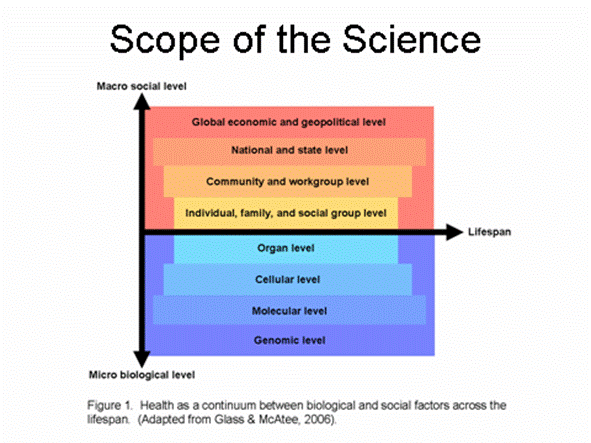
Much behavioral research on health assumes that individual action is an important (perhaps the most important) vehicle for understanding and changing social and health behaviors, but acknowledges that individuals are heavily influenced by actions occurring at other levels: from genomic, molecular, cellular, and organ systems, to family, workplace and community levels, to state, national, and global socioeconomic, environmental and geopolitical factors.�� Each additional layer of action requires more complex models.� To understand the relationships among these multiple levels, and to more fully elucidate their interactions, more sophisticated methodologies and technologies are needed.
Several fields have successfully developed or adopted a variety of systems approaches (e.g., biology, engineering, business).� The utilization of such approaches could prove promising in an interdisciplinary approach to addressing complex health problems.� For example, although numerous scientific advances have been made utilizing linear methods, the utilization of nonlinear methods holds additional promise.� Compared to linear methods, nonlinear, dynamical-systems methods require fewer a priori assumptions regarding the components of the system and fewer restrictions on how components may interact (Carello & Moreno, 2005).� Linear methods have been shown to have great value and further innovation in linear methodology could yield additional benefit. Yet there also is an unmet need for methodologies and technologies to more fully address the complexity of health problems in which behavioral and social factors play a role.� Complex problems may require expertise from a variety of disciplines and the integration of data at multiple levels to be addressed adequately. One purpose of this FOA is to address this need.��
Participating ICs invite qualified researchers to submit novel research grant applications designed to develop new/innovative measures, methods, and technologies that support interdisciplinary integration of human social and/or behavioral data with data from other disciplines at varying levels of analysis that may range from the level of the individual to the population to advance critical issues in health.� The goal of this Funding Opportunity Announcement (FOA) is to encourage research that will improve the quality and scientific power of data collected across multiple scientific disciplines, relevant to the missions of the NIH ICs.� For the purposes of this FOA, behavioral and social sciences research uses the NIH Office of Behavioral and Social Sciences definition, found at:� http://obssr.od.nih.gov/Content/About_OBSSR/BSSR_Definition/.� Supported research is expected to advance and integrate social and/or behavioral science with other discipline(s) as diverse as atmospheric science, biochemistry, biology, biomedicine, computational science, computer science, economics, engineering, geography, genetics, informatics/information science, mathematics, physics, physiology, and robotics.� Note that this list is meant to be illustrative, not all-inclusive.� Furthermore, the applicant must demonstrate the interdisciplinary nature of the particular disciplines being combined and provide a strong and compelling rationale for why this particular integration of disciplines will be fruitful.
Many of the concepts presented in this FOA were discussed at the NIH Roadmap Interdisciplinary Methodology and Technology Summit, held August 21-22, 2006.� A detailed description of the event including a webcast of this Summit, speakers� slide presentations, and background reading can be found at http://nihroadmap.nih.gov/interdisciplinary/summit0806/.� Additional information about priority research areas for behavioral and social sciences including systems approaches to addressing public health problems and interdisciplinary approaches, can be found at http://obssr.od.nih.gov/OBSSR10th/index.htm.
Research ScopeThe promise of interdisciplinary research lies in its power to establish new, shared conceptual frameworks among different disciplines to facilitate the creation of novel tools that enhance research on health and well-being. In this regard, this FOA will support the development of interdisciplinary research �tools,� or methodologies and technologies including measures, research designs, as well as analytic methods and techniques.� Specifically, this FOA will support the development of tools, methodologies and technologies, for use in research on humans, that integrate information, ideas, and concepts from the behavioral and/or social sciences with information, ideas, and concepts from other discipline(s).
This FOA is part of the NIH Roadmap for Medical Research.� As such, applications that address research needs that apply to more than one Institute or Center, or that fall in �gaps� between the missions of the Institutes or Centers, are being sought.� Applications must involve research teams.� Applications submitted by individual applicants, in the absence of a research team, will be deemed unresponsive.� Teams must be interdisciplinary, and must include behavioral and/or social scientists in addition to scientists of other discipline(s). Applicants must provide a strong and compelling rationale for why they chose the particular disciplines and how they expect the disciplines to work synergistically.�
Examples of research sought by this FOA include, but are not limited to, those described below. All examples are presumed to include interdisciplinary teams and approaches (including at least one behavioral or social scientist and social/behavioral approach), and to be relevant to health research, even if this is not evident in the example itself.
����������� Methodologies and/or technologies that improve or develop new approaches to nonlinear analysis e.g., recurrence quantification analysis, fractal analyses, systems dynamic modeling, agent-based modeling and other simulation techniques.
����������� Mathematical modeling of behavior (e.g., mathematical models to direct treatment of individuals over time and across development).
����������� Methodologies and/or technologies (especially unobtrusive, user-friendly, wearable devices and those utilizing WiFi technology) that improve measurement of behavioral phenomena in the �real-world� setting in which they typically occur and at the moment when the phenomena of interest actually occurs (i.e., ecological momentary assessment).� Methodologies and technologies related to co-occurring biological and behavioral processes are of particular interest.�
����������� Novel technologies to enable simultaneous measurement of behavioral and biological variables utilizing existing technologies, including, but not limited to, imaging probes, more sensitive noninvasive techniques, robotics or supercomputing networks.� For example, an application might propose to develop a predictive, dynamic simulation of diabetic metabolism, getting real-time input on glucose levels, activity levels, eating behavior and insulin dosing.� This in turn might lead to the development of interventional technologies with ability to warn patient/caregiver of upcoming danger.
����������� More fully refined and informative measures and/or diagnostic indicators of behavioral and/or psychiatric disorders, or of behavioral phenotypes that combine behavioral, emotional, cognitive and/or social indices at varying levels of analysis and across development, with biological markers, for use in assessment in interdisciplinary research, such as genetics research.
����������� Methodologies and/or technologies that integrate neuroscience with the macro (i.e., social) environment.� For example, those that address research questions such as: how do neighborhood conditions (e.g., violence, drug dealing, density, green space) impact brain development? Are differences in brain morphology or function due to genetic factors, environmental factors, or some interplay of the two?
����������� Methodologies and/or technologies that facilitate biosocial research designed to understand and possibly redefine the concept of socioeconomic status as it relates to health behavior and biological health outcomes.� For example, an application might propose to develop new, multidimensional measures that expand on existing measures of socioeconomic status, and that capture health, behavior, and biological outcomes.
����������� Methodologies and/or technologies that facilitate understanding of biological and neurobiological effects of behavioral interventions.
����������� Technologies that induce behavioral, emotional, and/or social experiences along with measures to facilitate the assessment of these experiences and co-occurring biological phenomena.
����������� Methodologies and/or technologies that facilitate the study of gene/environment interactions on a large scale, especially those that explore the bidirectional nature of these relationships (i.e., not only how physical, behavioral or social environments impact gene expression and the biological mechanisms that mediate these relationships, but also how genes impact selection to particular environments and the underlying behavioral mechanisms that may mediate such relationships).
����������� Methodologies and technologies to elucidate how gene-behavior-environment interactions influence health, including tools to improve our understanding of bidirectional relationships between factors at various levels of analysis (e.g., the effects of genes on the selection to particular environments as well as the impact of environmental exposure on gene expression).
����������� Methodologies and/or technologies that facilitate integration of sub-individual biological and genetic data with individual level behavioral data (i.e., data derived from measures of actual behavior as opposed to self-report or others� reports of behavior).
����������� Methodologies and/or technologies that improve or facilitate integration and analysis of large data sets, (e.g., integrating behavioral data with genomic or ecologic data, or data resulting from frequent observational or streaming data such as from ecological momentary assessment).
����������� Methodologies and/or technologies that improve or facilitate the capture, analysis, and interpretation of social network data at a variety of levels.
����������� Methodologies and/or technologies that facilitate analysis of data integrated across multiple levels within the micro- to macro continuum (from sub-individual, including genetic data; to individual level, including behavior; to neighborhood and community level, including social interactions; to population level, including policies, economics, media influences).�
����������� Methodologies and/or technologies that facilitate visualization of data integration across levels and time.
����������� Development of methodologies and technologies to improve integration of molecular epidemiology data with behavioral and social science data (e.g., survey data).� Such methodologies and technologies would help inform and cross-check our understanding of network dynamics and structure. For example, molecular epidemiology can recognize particular strains of a disease as they have passed through the network of contacts, recognize cases of co-infection/multiple infection, and can in certain diseases even shed light on how long an individual has been infected with a disease.� Coupling this with the data collected by survey instruments could help to produce better results from simulation and modeling (e.g., infectious disease spread, understanding the obesity epidemic).
����������� Methodologies and/or technologies that improve or facilitate the integration of environmental data (e.g., geospatial, information, local policy, economic status of community, or availability of health care in the community), with behavioral, biological, genetic, and other health-related data at the individual level.�
����������� Methodologies and/or technologies that foster the growth of the emerging field of populomics (i.e., the study of community-wide risk profiles; see Gibbons, 2005). For example, an application might propose to develop a methodology or technology designed to integrate data across multiple levels of analysis, e.g., geospatial, policy, economic, health care, or biological, with behavioral or social data at the neighborhood or community level. It is recognized that there is great variability in the characteristics of international communities and neighborhoods. Tools are sought to capture and analyze these differences.
����������� Methodologies and/or technologies that improve our understanding of syndemics (synergistic or co-occurring epidemics), by using social-level data integrated with individual behavioral and/or biological data. This could include methodologies or technologies to improve integration and interpretation of data from environmental sensors with data from other levels.
����������� Methodologies and/or technologies that increase availability of systems dynamic modeling, agent-based modeling or other modeling or simulation methodologies designed to integrate social and behavioral data with data from other areas (e.g., genetics, biology, economics, media campaigns, policy).
����������� Methodologies and/or technologies that improve or extend social networking analysis tools to enable integration of social network data with disease data.� For example, integrating the use of assays for identification of recent infection [RNA PCR, STARHS] with social network and ethnographic techniques for identifying the context of recent infection (e.g., venues where anonymous/casual contacts meet). Such methodologies and technologies could further the current understanding of demographic structure and/or social networks to shed light on the diffusion of infectious agents and guide the design and implementation of social policies aimed at preventing or reducing epidemics.�
����������� Methodologies and/or technologies that enable prediction of potential consequences of policy changes on behavior and health, particularly in the area of research on disparities in health outcomes among different socioeconomic and racial/ethnic groups.
����������� Methodologies and/or technologies that facilitate research on initiation and maintenance of long term behavior change.� For example, technology that enables long term monitoring of smokers, obese persons, or patients with chronic disorders; or methodology that informs the sequential decision making by clinicians when managing these disorders; or measures designed to accurately capture repeated measurement of patient outcomes.
����������� Methodologies and/or technologies that facilitate strategies to mask identifying personal information while retaining sufficient information to yield interpretable and meaningful results for secondary data analysis.� For example, devising algorithms to create synthetic populations with the same statistical properties as the real population.
����������� Methodologies and/or technologies that improve our understanding of how ongoing demographic changes, such as migration and alterations in the distribution of economic resources, associate with developing patterns of health risks and service access.� Ideally, new methods of observation and data analysis will support claims about causal relationships, not just correlations, between social factors and health status.�
����������� Methodologies and/or technologies that improve or facilitate integration of public health research with existing community-based indicator systems and data warehouses that have evolved from research in social work, applied economics, demography, and urban planning.� Doing so would build on evolving efforts to understand ecological and longitudinal trends in economics and population dynamics. It would also pave the way for novel approaches to modeling patterns of infectious and chronic disease as well as surveillance, prevention, and treatment.
����������� Methodologies and/or technologies that improve our understanding of how communities and neighborhoods trigger biological processes in humans that relate to health disparities in the population.
����������� Methodologies and/or technologies to improve our understanding of the bi-directional relationship between developmental processes at the individual and macro (societal) levels.� For example, those that address such research questions as does community capacity building impact children�s brain and behavioral development?
����������� Methodologies and/or technologies that improve or facilitate simultaneous location of multiple individuals and examine network structure and dynamics (frequency and duration of individual contact, degree of mixing).� Social interaction data could combine with location-based information, time stamps, and activity level information to provide a richer picture of social networks.
����������� Methodologies and/or technologies that improve or facilitate information visualization via open source software to integrate data at multiple levels of analysis and/or facilitate the analysis of large data sets.
����������� Technology to integrate video data with large scale survey data to provide access that protects participant confidentiality and permits qualified parties with appropriate permissions to analyze this data.� Such technology would illuminate the details of human interaction and context and would require content experts in the social and behavioral sciences, along with information technologists, computer engineers, and videographers.�
ReferencesCarello, C., & Moreno, M.A... (2005). Why Non-linear Methods? In M. A. Riley & G. C. Van Orden (Eds.), Tutorials in contemporary nonlinear methods for the behavioral sciences (pp. 353-400).
Retrieved September 1, 2006, from http://www.nsf.gov/sbe/bcs/pac/nmbs/nmbs.jsp
Gibbons, M.C. (2005).�� A Historical Overview of Health Disparities and the Potential of eHealth Solutions Journal of Medical Internet Research 7(5) e50.
Glass, T.A. & McAtee, M.J. (2006).� Behavioral science at the crossroads in public health: Extending horizons, envisioning the future.� Social Science & Medicine 62(7) 1650-1671.
See Section VIII, Other Information - Required Federal
Citations, for policies related to this announcement.
Section
II. Award Information
1. Mechanism of Support
This Funding Opportunity Announcement (FOA) will use the NIH
Exploratory/Developmental Research Grant (R21) award mechanism. � The applicant will be solely responsible for planning,
directing, and executing the proposed project.
This FOA uses �Just-in-Time� information concepts. It also uses the modular as well as the non-modular budget formats (see the �Modular Applications and Awards� section of the NIH Grants Policy Statement. Specifically, if you are submitting an application with direct costs in each year of $250,000 or less (excluding consortium Facilities and Administrative [F&A] costs), use the PHS398 Modular Budget component provided in the SF424 (R&R) Application Package and SF424 (R&R) Application Guide (see specifically Section 5.4, �Modular Budget Component,� of the Application Guide).
All foreign applicants must complete and submit budget requests using the Research & Related Budget component found in the application package for this FOA. See NOT-OD-06-096, August 23, 2006.
Competing Renewals are not allowed.
2. Funds Available
Because the nature and scope of the proposed research will vary from
application to application, it is anticipated that the size and duration of
each award will also vary. Although the financial plans of the Institutes and
Centers (ICs) provide support for this program, awards pursuant to this funding
opportunity are contingent upon the availability of funds and the submission of
a sufficient number of meritorious applications.
NIH intends to commit approximately $3 million dollars in FY2007 to fund approximately 10 new grants in response to this FOA. The total project period for an applications submitted in response to this funding opportunity may not exceed 4years. Total costs are limited to $1,200,000 over a four-year period, with no more than $300,000 in total costs allowed in any single year. The earliest anticipated start date for awards is September 15, 2007.�
NIH
grants policies as described in the NOT-OD-05-004,
November 2, 2004.
Section
III. Eligibility Information
1. Eligible Applicants
1.A. Eligible Institutions
You may submit an application(s) if your institution/organization has any of the following characteristics:
- Public/State Controlled Institution of Higher Education
- Private Institution of Higher Education
- Nonprofit with 501(c)(3) IRS Status (Other than Institution of Higher Education)
- Nonprofit without 501(c)(3) IRS Status (Other than Institution of Higher Education)
- Small Business
- For-Profit Organization (Other than Small Business)
- State Government
- U.S. Territory or Possession
- Indian/Native American Tribal Government (Federally Recognized)
- Indian/Native American Tribal Government (Other than Federally Recognized)
- Indian/Native American Tribally Designated Organization
- Non-domestic (non-U.S.) Entity (Foreign Organization)
- Hispanic-serving Institution
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Regional Organization
- Eligible agencies of the Federal government; Faith-based or community based organizations
1.B. Eligible Individuals
Any individual with the skills, knowledge, and resources necessary to carry out the proposed research as the Project Director/Principal Investigator (PD/PI) is invited to work with his/her organization to develop an application for support. Individuals from underrepresented racial and ethnic groups as well as individuals with disabilities are always encouraged to apply for NIH support.
2.
Cost Sharing or Matching
This program does not require cost sharing as defined in the current NIH
Grants Policy Statement.
3. Other-Special
Eligibility Criteria
Applicants must propose interdisciplinary teams to
accomplish the proposed research.�
Applicants may submit more than one application, provided
each application is scientifically distinct.
� ��������� Trans-NIH focus. Because this FOA is part of the NIH Roadmap initiative, applications should address research needs that apply to more than one Institute or Center, or that �fall between� the missions of the Institutes or Centers.�
����������� Interdisciplinary teams. Applications must involve research teams made up of more than one discipline.� Applications submitted by individual applicants, in the absence of a research team, will be deemed unresponsive. Teams must include behavioral and/or social scientists in addition to scientists of other discipline(s).
NIH recognizes that multidisciplinary approaches may be a necessary initial step in furthering interdisciplinary approaches.� However, for the purposes of this FOA, proposals that involve multiple disciplines, but do not completely achieve integration of human behavioral or social science with other scientific discipline(s), will be considered non-responsive and will not be reviewed.� Applications that bring together more than two disciplines and or bring together disciplines that do not typically work together are welcome.� All applications must provide a strong rationale for choosing the disciplines that are brought together.�
����������� Interdisciplinary approach. Applicants should expressly highlight the interdisciplinary aspects of their application, and include a description of the way(s) in which the behavioral and/or social sciences will integrate with other different disciplines in performing the proposed work.
����������� Human focus.� The project(s) proposed should pertain to human problems and be human focused. While animal research may inform the proposed work and animal researchers may be part of the research team, applications with a focus primarily on animal research will be deemed unresponsive.
Applicants are strongly encouraged to read the FOA thoroughly and carefully and to discuss their plans with the relevant program staff well in advance of the application receipt date (see Section VII. Agency Contacts).� There will be a conference call open to all persons interested in responding to this FOA.� The purpose of the call will be to provide technical assistance for the application process and to answer any questions regarding the application process and responsiveness issues.� Please check the NIH Guide to Grants and Contracts for a notice announcing the details of this call. �
Section IV. Application and Submission Information
To download a SF424 (R&R) Application Package and
SF424 (R&R) Application Guide for completing the SF424 (R&R) forms for
this FOA, link to http://www.grants.gov/Apply/ and follow the directions provided on that Web site.
A one-time registration is required for institutions/organizations at both:
- Grants.gov (http://www.grants.gov/GetStarted) and
- eRA Commons (http://era.nih.gov/ElectronicReceipt/preparing.htm)
PDs/PIs should work with their institutions/organizations to make sure they are registered in the eRA Commons.
Several additional separate actions are required before an applicant institution/organization can submit an electronic application, as follows:
1) Organizational/Institutional Registration in Grants.gov/Get Started
- Your organization will need to obtain a Data Universal Number System (DUNS) number and register with the Central Contractor Registration (CCR) as part of the Grants.gov registration process.
- If your organization does not have a Taxpayer Identification Number (TIN) or Employer Identification Number (EIN), allow for extra time. A valid TIN or EIN is necessary for CCR registration.
- The CCR also validates the EIN against Internal Revenue Service records, a step that will take an additional one to two business days.
- Direct
questions regarding Grants.gov registration to:
Grants.gov Customer Support
Contact Center Phone: 800-518-4726
Business Hours: M-F 7:00 a.m. - 9:00 p.m. Eastern Time
Email [email protected]
2) Organizational/Institutional Registration in the eRA Commons
- To find out if an organization is already Commons-registered, see the "List of Grantee Organizations Registered in NIH eRA Commons.�
- Direct
questions regarding the Commons registration to:
eRA Commons Help Desk
Phone: 301-402-7469 or 866-504-9552 (Toll Free)
TTY: 301-451-5939
Business hours M-F 7:00 a.m. � 8:00 p.m. Eastern Time
Email [email protected]
3) Project Director/Principal Investigator (PD/PI) Registration in the NIH eRA Commons: Refer to the NIH eRA Commons System (COM) Users Guide.
- The individual designated as the PD/PI on the application must also be registered in the NIH eRA Commons. It is not necessary for PDs/PIs to register with Grants.gov.
- The PD/PI must hold a PD/PI account in the Commons and must be affiliated with the applicant organization. This account cannot have any other role attached to it other than the PD/PI.
- This registration/affiliation must be done by the Authorized Organization Representative/Signing Official (ARO/SO) or their designee who is already registered in the Commons.
- Both the PD/PI and AOR/SO need separate accounts in the NIH eRA Commons since both are authorized to view the application image.
Note that if a PD/PI is also an NIH peer-reviewer with an Individual DUNS and CCR registration, that particular DUNS number and CCR registration are for the individual reviewer only. These are different than any DUNS number and CCR registration used by an applicant organization. Individual DUNS and CCR registration should be used only for the purposes of personal reimbursement and should not be used on any grant applications submitted to the Federal Government.
Several of the steps of the registration process could take four weeks or more. Therefore, applicants should immediately check with their business official to determine whether their organization/institution is already registered in both Grants.gov and the Commons. The NIH will accept electronic applications only from organizations that have completed all necessary registrations.
1. Request Application Information
Applicants must download the SF424 (R&R)
application forms and SF424 (R&R) Application Guide for this FOA through Grants.gov/Apply.
Note: Only the forms package
directly attached to a specific FOA can be used. You will not be able to use
any other SF424 (R&R) forms (e.g., sample forms, forms from another FOA),
although some of the "Attachment" files may be useable for more than
one FOA.
For further assistance, contact GrantsInfo: Telephone
301-710-0267, Email: [email protected].
Telecommunications for the hearing impaired: TTY
301-451-0088.
2. Content and Form of Application Submission
Prepare all applications using the SF424 (R&R) application forms and in accordance with the SF424 (R&R) Application Guide for this FOA through Grants.gov/APPLY.
The SF424 (R&R) Application Guide is critical to submitting a complete and accurate application to NIH. There are fields within the SF424 (R&R) application components that, although not marked as mandatory, are required by NIH (e.g., the �Credential� log-in field of the �Research & Related Senior/Key Person Profile� component must contain the PD/PI�s assigned eRA Commons User ID). Agency-specific instructions for such fields are clearly identified in the Application Guide. For additional information, see �Frequently Asked Questions � Application Guide, Electronic Submission of Grant Applications.�
The SF424 (R&R) application is comprised of data arranged in separate components. Some components are required, others are optional. The forms package associated with this FOA in Grants.gov/APPLY will include all applicable components, required and optional. A completed application in response to this FOA will include the following components:
Required
Components:
SF424 (R&R) (Cover component)
Research & Related Project/Performance Site
Locations
Research & Related Other Project Information
Research & Related Senior/Key Person
PHS398 Cover Page Supplement
PHS398 Research Plan
PHS398 Checklist
PHS398 Modular Budget (required for U.S. applications)
Research & Related Budget (required for foreign
applications)
Optional Components:
PHS398 Cover Letter File
Research & Related Subaward Budget Attachment(s)
Form
Foreign Organizations (Non-domestic (non-U.S.) Entity)
NIH policies concerning grants
to foreign (non-U.S.) organizations can be found in the NIH Grants Policy
Statement at: https://grants.nih.gov/archive/grants/policy/nihgps_2003/index.htm#_Toc54600260.
Applications from foreign organizations must:
- Request budgets in U.S. dollars.
- Prepare detailed budgets for all applications (that is, complete the Research & Related Budget component of the SF424 (R&R) application forms � not the PHS398 Modular Budget component).� See NOT-OD-06-096
- Charge back of customs and import fees is not allowed.
- Format: Every effort should be made to comply with the format specifications, which are based upon a standard U.S. paper size of 8.5� x 11� within each PDF.
- Funds for up to 8% administrative costs (excluding equipment) may be requested. See NOT-OD-01-028, March 29, 2001.
- Organizations must comply with Federal/NIH policies on human subjects, animals, and biohazards.
- Organizations must comply with Federal/NIH biosafety and biosecurity regulations. See Section VI.2., �Administrative and National Policy Requirements.�
Proposed research should provide special opportunities for furthering research programs through the use of unusual talent, resources, populations, or environmental conditions in other countries that are not readily available in the United States or that augment existing U.S. resources.
SPECIAL INSTRUCTIONS��
Applications with Multiple PDs/PIs
When multiple PDs/PIs are proposed, NIH requires one PD/PI to be designated as the "Contact� PI, who will be responsible for all communication between the PDs/PIs and the NIH, for assembling the application materials outlined below, and for coordinating progress reports for the project. The contact PD/PI must meet all eligibility requirements for PD/PI status in the same way as other PDs/PIs, but has no other special roles or responsibilities within the project team beyond those mentioned above.
Information for the Contact PD/PI should be entered in item 15 of the SF424 (R&R) Cover component. All other PDs/PIs should be listed in the Research & Related Senior/Key Person component and assigned the project role of �PD/PI.� Please remember that all PDs/PIs must be registered in the eRA Commons prior to application submission. The Commons ID of each PD/PI must be included in the �Credential� field of the Research & Related Senior/Key Person component. Failure to include this data field will cause the application to be rejected.
All projects proposing Multiple PDs/PIs will be required to include a new section describing the leadership of the project.
Multiple PD/PI Leadership Plan: For applications designating multiple PDs/PIs, a new section of the research plan, entitled �Multiple PD/PI Leadership Plan� (section 14 of the Research Plan Component in the SF424 (R&R) or Section I of the Research Plan in the PHS 398), must be included. A rationale for choosing a multiple PD/PI approach should be described.� The governance and organizational structure of the leadership team and the research project should be described, including communication plans, process for making decisions on scientific direction, and procedures for resolving conflicts. The roles and administrative, technical, and scientific responsibilities for the project or program should be delineated for the PDs/PIs and other collaborators.�
If budget allocation is planned, the distribution of resources to specific components of the project or the individual PDs/PIs should be delineated in the Leadership Plan.� In the event of an award, the requested allocations may be reflected in a footnote on the Notice of Award.
Applications Involving a Single Institution
When all PDs/PIs are within a single institution, follow the instructions contained in the SF424 (R&R) Application Guide.
Applications Involving Multiple Institutions
When multiple institutions are involved, one institution must be designated as the prime institution and funding for the other institution(s) must be requested via a subcontract to be administered by the prime institution. When submitting a detailed budget, the prime institution should submit its budget using the Research & Related Budget component. All other institutions should have their individual budgets attached separately to the Research & Related Subaward Budget Attachment(s) Form. See Section 4.8 of the SF424 (R&R) Application Guide for further instruction regarding the use of the subaward budget form.
When submitting a modular budget, the prime institution completes the PHS398 Modular Budget component only. Information concerning the consortium/subcontract budget is provided in the budget justification. Separate budgets for each consortium/subcontract grantee are note required when using the Modular budget format. See Section 5.4 of the Application Guide for further instruction regarding the use of the PHS398 Modular Budget component.
3.
Submission Dates and Times
See Section IV.3.A for
details.
3.A.
Submission, Review, and Anticipated Start Dates
Opening Date: January 23, 2007 (Earliest date an application may be
submitted to Grants.gov)
Letters
of Intent Receipt Date(s): January 23, 2007
Application
Submission/Receipt Date(s): February 23, 2007
Peer Review
Date(s): June 2007
Council Review
Date(s): August 2007 �
Earliest
Anticipated Start Date(s): September 30, 2007
3.A.1. Letter of Intent
Prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed research.
- Name, address, and telephone number of the PD/PI.
- Names of other key personnel.
- Participating institutions.
- Number and title of this funding opportunity.
Although a
letter of intent is not required, is not binding, and does not enter into the
review of a subsequent application, the information that it contains allows IC
staff to estimate the potential review workload and plan the review.
The letter of intent is to be sent by the date listed
in Section IV.3.A.
The letter of intent should be sent to:
RM-07-004
Director
Office of Extramural Affairs
National
Institute on Drug Abuse, NIH, DHHS
6101
Executive Boulevard, Suite 220, MSC
8401
Bethesda, Maryland� 20892-8401
Telephone:�
(301) 443-2755
FAX:�
(301) 443-0538
Email:� [email protected]
3.B. Submitting an Application Electronically to the
NIH
To submit an application in response to this FOA, applicants should access this
FOA via http://www.grants.gov/Apply and follow steps 1-4. Note: Applications must only be submitted
electronically. PAPER APPLICATIONS WILL NOT BE ACCEPTED.
In order to expedite the review, applicants are requested to notify the National Institute on Drug Abuse Referral Office by email ([email protected]) when the application has been submitted.� Please include the FOA number and title, PD/PI name, key personnel, and title of the application.
3.C.
Application Processing
Applications may be submitted on or after
the opening date and must be successfully received by Grants.gov no
later than 5:00 p.m. local time (of the
applicant institution/organization) on the
application submission/receipt date(s). (See Section IV.3.A. for all dates.) If an application is not submitted by the receipt
date(s) and time, the application may be delayed in the review process or not
reviewed.
Once an application package has been successfully submitted through Grants.gov, any errors have been addressed, and the assembled application has been created in the eRA Commons, the PD/PI and the Authorized Organization Representative/Signing Official (AOR/SO) have two business days to view the application image.
- If everything is acceptable, no further action is necessary. The application will automatically move forward for processing by the Division of Receipt and Referral, Center for Scientific Review, NIH, after two business days.
- Prior to the submission deadline, the AOR/SO can �Reject� the assembled application and submit a changed/corrected application within the two day viewing window. This option should be used if the AOR/SO determines that warnings should be addressed. Reminder: warnings do not stop further application processing. If an application submission results in warnings (but no errors) it will automatically move forward after two business days if no action is taken.� Please remember that some warnings may not be applicable or may need to be addressed after application submission.
- If the two day window falls after the submission deadline, the AOR/SO will have the option to �Reject� the application if, due to an eRA Commons or Grants.gov system issue, the application does not correctly reflect the submitted application package (e.g., some part of the application was lost or didn�t transfer correctly during the submission process). The AOR/SO should first contact the eRA Commons Helpdesk to confirm the system error, document the issue, and determine the best course of action. NIH will not penalize the applicant for an eRA Commons or Grants.gov system issue.
- If the AOR/SO chooses to �Reject� the image after the submission deadline for a reason other than an eRA Commons or Grants.gov system failure, a changed/corrected application still can be submitted but it will be subject to the NIH late policy guidelines and may not be accepted. The reason for this delay should be explained in the cover letter attachment.
- Both the AOR/SO and PD/PI will receive e-mail notifications when the application is rejected or the application automatically moves forward in the process after two days.
Upon receipt, applications will
be evaluated for completeness by the Center for Scientific Review, NIH.
Incomplete applications will not be reviewed.
There will be an acknowledgement of receipt of
applications from Grants.gov and the Commons. Information related to the
assignment of an application to a Scientific Review Group is also in the Commons.
Note: Since email can be unreliable, it is the responsibility of the applicant to check periodically on their application status in the Commons.
The NIH will not accept any application in response to this funding opportunity that is essentially the same as one currently pending initial review, unless the applicant withdraws the pending application. However, when a previously unfunded application, originally submitted as an investigator-initiated application, is to be submitted in response to a funding opportunity, it is to be prepared as a NEW application. That is, the application for the funding opportunity must not include an Introduction describing the changes and improvements made, and the text must not be marked to indicate the changes from the previous unfunded version of the application.
4. Intergovernmental Review
This initiative is not subject to intergovernmental
review.
5.
Funding Restrictions
All NIH awards are subject to the terms and
conditions, cost principles, and other considerations described in the NIH Grants
Policy Statement.
Pre-Award Costs are allowable. A grantee may, at
its own risk and without NIH prior approval, incur obligations and expenditures
to cover costs up to 90 days before the beginning date of the initial budget
period of a new award if such costs: are necessary to conduct the project, and
would be allowable under the grant, if awarded, without NIH prior approval. If
specific expenditures would otherwise require prior approval, the grantee must
obtain NIH approval before incurring the cost. NIH prior approval is required
for any costs to be incurred more than 90 days before the beginning date of the
initial budget period of a new award.
The incurrence of pre-award costs in anticipation of a
competing or non-competing award imposes no obligation on NIH either to make
the award or to increase the amount of the approved budget if an award is made
for less than the amount anticipated and is inadequate to cover the pre-award
costs incurred. NIH expects the grantee to be fully aware that pre-award costs
result in borrowing against future support and that such borrowing must not
impair the grantee's ability to accomplish the project objectives in the
approved time frame or in any way adversely affect the conduct of the project. See
the NIH Grants
Policy Statement.
6. Other Submission
Requirements
PD/PI
Credential (e.g., Agency Login)
The NIH requires the PD/PI to fill in his/her Commons User ID in the �PROFILE � Project Director/Principal Investigator� section, �Credential� log-in field of the �Research & Related Senior/Key Person Profile� component. The applicant organization must include its DUNS number in its Organization Profile in the eRA Commons. This DUNS number must match the DUNS number provided at CCR registration with Grants.gov. For additional information, see �Registration FAQs � Important Tips -- Electronic Submission of Grant Applications.�
Organizational DUNS
The applicant organization must include its DUNS number in its Organization Profile in the eRA Commons. This DUNS number must match the DUNS number provided at CCR registration with Grants.gov. For additional information, see �Frequently Asked Questions � Application Guide, Electronic Submission of Grant Applications.�
Warning: Please be sure that you observe the direct cost, project period, and page number limitations specified above for this FOA. Application processing may be delayed or the application may be rejected if it does not comply with these requirements.
Research Plan Component Sections
While each section of the Research Plan component needs to be uploaded separately as a PDF attachment, applicants are encouraged to construct the Research Plan component as a single document, separating sections into distinct PDF attachments just before uploading the files. This approach will enable applicants to better monitor formatting requirements such as page limits. All attachments must be provided to NIH in PDF format, filenames must be included with no spaces or special characters, and a .pdf extension must be used.
All application instructions outlined in the SF424 (R&R) Application Guide are to be followed, incorporating "Just-in-Time" information concepts, and with the following requirements for R21 applications:
- Items 2-5 of the Research Plan component of the R21 application may not exceed 15 pages, including tables, graphs, figures, diagrams, and charts.
- Preliminary data are not required but may be included if available.
- Renewal (formerly "competing continuation" or "Type 2") applications are not permitted.
Appendix Materials
The following materials may be included in the Appendix:
- Up tofive publications, manuscripts (accepted for publication), abstracts, patents, or other printed materials directly relevant to the proposed project. Do not include manuscripts submitted for publication. See NOT-OD-06-053.�
- Publications in press: Include only a publication list with a link to the publicly available on-line journal article or the NIH PubMed Central (PMC) submission identification number. Do not include the entire article.
- Manuscripts accepted for publication but not yet published: The entire article may be submitted electronically as a PDF attachment.
- Manuscripts published but a publicly available online journal link is not available: The entire article may be submitted electronically as a PDF attachment.
- Surveys, questionnaires, data collection instruments, clinical protocols, and informed consent documents.
- Graphic images of gels, micrographs, etc. provided that the image (may be reduced in size) is also included within the (stated) page limit of Items 2-5 of the Research Plan component. No images may be included in the Appendix that are not also represented within the Research Plan.
Do not use the Appendix to circumvent the page limitations of the Research Plan component. An application that does not observe the relevant policies and procedures may be delayed in the review process.
Foreign Applications (Non-domestic (non-U.S.) Entity)
- Indicate how the proposed project has specific relevance to the mission and objectives of the IC and has the potential for significantly advancing the health sciences in the United States.�
NIH believes that data sharing is essential for expedited translation of research �results into knowledge, products, and procedures to improve human health. Therefore, NIH expects and supports the timely release and sharing of final research data from NIH-supported studies for use by other researchers. All applicants responding to this FOA are expected to include a plan for data sharing with a full description of any limitations on data sharing, or state why data sharing is not possible.
NIH recognizes that data sharing may be complicated or limited, in some cases, by institutional policies, local IRB rules, as well as local, state and Federal laws and regulations, including the Privacy Rule. As NIH has stated in other Guide announcements, the rights and privacy of people who participate in NIH-sponsored research must be protected at all times. Thus, data intended for broader use should be free of identifiers that would permit linkages to individual research participants and variables that could lead to deductive disclosure of the identity of individual subjects. When data sharing is limited, applicants should explain such limitations in their data sharing plans. Additional information on NIH data sharing policies is available at: https://grants.nih.gov/grants/policy/data_sharing/.
The precise content of the data-sharing plan may vary, depending on the data being collected, whether or not new technology is being developed, and how the investigator is planning to share the data. Applicants who are planning to share data may wish to describe briefly the expected schedule for data sharing, the format of the final dataset, the documentation to be provided, whether or not any analytic tools will also be provided, whether or not a data-sharing agreement will be required and, if so, a brief description of such an agreement (including the criteria for deciding who can receive the data and whether or not any conditions will be placed on their use), and the mode of data sharing (e.g., under their own auspices by mailing a disk or posting data on their institutional or personal website, through a data archive or enclave). Investigators choosing to share under their own auspices may wish to enter into a data-sharing agreement. References to data sharing may also be appropriate in other sections of the application.
The reasonableness of the data sharing plan or the rationale for not sharing research data may be assessed by the reviewers. However, reviewers will not factor the proposed data sharing plan into the determination of scientific merit or the priority score. Program staff will be responsible for overseeing the data sharing policy and for assessing the appropriateness and adequacy of the proposed data-sharing plan. Applicants are encouraged to discuss their data sharing plan with their program contact prior to submitting their application.
Sharing Research ResourcesNIH
policy requires that grant awardee recipients make unique research resources
readily available for research purposes to qualified individuals within the
scientific community after publication (See the NIH Grants Policy Statement� https://grants.nih.gov/archive/grants/policy/nihgps_2003/index.htm#_Toc54600131).
Investigators responding to this funding opportunity should include a sharing
research resources plan addressing how unique research resources will be shared
or explain why sharing is not possible.
The adequacy of the resources sharing plan and any
related data sharing plans will be considered by Program staff of the funding
organization when making recommendations about funding applications. The
effectiveness of the resource sharing will be evaluated as part of the
administrative review of each Non-Competing Grant
Progress Report (PHS 2590). See Section VI.3.,
�Reporting.�
Any software developed under this FOA should be freely available to other biomedical researchers in the non-profit sector. �
Section V. Application Review Information
1. Criteria
Only the review
criteria described below will be considered in the review process.
2. Review and
Selection Process
Upon receipt, applications
will be evaluated for responsiveness by the NIH Roadmap Project Team. �
Incomplete and/or non-responsive applications will not be reviewed.
It is strongly recommended that prospective applicants contact the staff person listed under INQUIRIES early in the planning phase of the application.� Such contact will help ensure that applications are responsive to the overall intent of this award.
Applications
that are complete and responsive to the FOA will be evaluated for scientific
and technical merit by an appropriate peer review group convened by the National Institute on Drug Abuse in accordance with the review criteria stated below.
As part of the initial merit review, all applications will:
- Undergo a selection process in which only those applications deemed to have the highest scientific merit, generally the top half of applications under review, will be discussed and assigned a priority score.
- Receive a written critique.
- Receive a second level of review by the National Advisory Council on Drug Abuse.
Applications submitted in response to this funding opportunity will compete for available funds with all other recommended applications. The following will be considered in making funding decisions:
- Scientific merit of the proposed project as determined by peer review.
- Availability of funds.
- Relevance to program priorities.
The goals of NIH supported research are to advance our understanding of biological systems, to improve the control of disease, and to enhance health. In their written critiques, reviewers will be asked to comment on each of the following criteria in order to judge the likelihood that the proposed research will have a substantial impact on the pursuit of these goals. Each of these criteria will be addressed and considered in assigning the overall score, weighting them as appropriate for each application.
- Significance
- Approach
- Innovation
- Investigator
- Environment
The NIH R21 exploratory/developmental grant is a mechanism for supporting novel scientific ideas or new model systems, tools, or technologies that have the potential to significantly advance our knowledge or the status of health-related research.� Because the Research Plan component is limited to 15 pages, an exploratory/developmental grant application need not have extensive background material or preliminary information or data as one might normally expect in an R01 application.� Accordingly, reviewers will focus their evaluation on the conceptual framework, the level of innovation, and the potential to significantly advance our knowledge or understanding.� Appropriate justification for the proposed work can be provided through literature citations, data from other sources, or, when available, from investigator-generated data.� Preliminary data are not required for R21 applications; however, they may be included if available.
NIH is the steward of medical and behavioral research for the Nation. Its mission is science in pursuit of fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to extend healthy life and reduce the burdens of illness and disability. In their written critiques, reviewers will be asked to comment on each of the following criteria in order to judge the likelihood that the proposed research will have a substantial impact on the pursuit of these goals. Each of these criteria will be addressed and considered in assigning the overall score, weighting them as appropriate for each application.
Note that an application does not need to be strong in all
categories to be judged likely to have major scientific impact and thus deserve
a high priority score.
Significance: Will this study contribute to improving science to foster public health?
Is there a clear trans-NIH focus?� If the aims of the application are achieved,
how will scientific knowledge or clinical practice be advanced? What will be
the effect of this work on concepts, methods, technologies, treatments,
services, or preventative interventions?� Does the application address the
goals and objectives outlined in the FOA?
Approach: Is the approach interdisciplinary?� Does the proposed approach
involve the integration of multiple levels of science? Are the conceptual framework, design, methods, and analyses adequately
developed, well integrated, well reasoned, and appropriate to the aims of the
project? Is the approach feasible?� Is the approach rigorous?� Does the
application acknowledge potential problem areas and consider alternative
tactics? �For applications designating multiple PDs/PIs, is the leadership
approach, including the designated roles and responsibilities, governance and
organizational structure consistent with and justified by the aims of the
project and the expertise of each of the PDs/PIs?
Innovation: Is the project original and innovative in its interdisciplinary approach?� Does the project challenge existing paradigms or critical barriers to advancing the public health? Does the project develop or employ novel interdisciplinary concepts, approaches, methodologies, tools, and/or technologies?� Does the project demonstrate innovative ways to integrate multiple levels of science?�
Investigators: Does the team
include behavioral and/or social scientists, in addition to scientist(s) from
one or more other disciplines?� Is the investigative team sufficiently
interdisciplinary, and appropriately trained and well suited to carry out this
work?� Is there a coherent and compelling rationale for bringing together the
disciplines represented on the investigative team?� Does the investigative team
bring complementary and integrated expertise to the project (if applicable)?�
Is the work proposed appropriate to the experience level of the PD/PI and other
researchers? For established interdisciplinary teams, is there sufficient
evidence that the team has collaborated successfully, and will continue to do
so?� For newly-formed interdisciplinary teams, is there sufficient evidence
that the team can collaborate successfully?
Environment: Does the scientific environment (or environments) in which
the work will be done contribute to the probability of success? Do the proposed
studies benefit from unique features of the scientific environments, or subject
populations, or employ useful collaborative arrangements? Is there evidence of
institutional support?
2.A.
Additional Review Criteria:
In addition to the above criteria, the following items
will continue to be considered in the determination of scientific merit and the
priority score:
Protection of Human Subjects from Research Risk: The involvement of human subjects and protections from research risk relating
to their participation in the proposed research will be assessed. See
item 6 of the Research Plan component of the SF424 (R&R).
Inclusion of Women, Minorities and Children in Research: The adequacy of
plans to include subjects from both genders, all racial and ethnic groups (and
subgroups), and children as appropriate for the scientific goals of the
research will be assessed. Plans for the recruitment and retention of subjects
will also be evaluated. See item 7 of the Research Plan component of the
SF424 (R&R).
Care and Use of Vertebrate Animals in Research: If vertebrate animals
are to be used in the project, the five items described under item 11 of the
Research Plan component of the SF424 (R&R) will be assessed.
Biohazards: If materials or procedures are proposed that are potentially
hazardous to research personnel and/or the environment, determine if the
proposed protection is adequate.�
2.B.
Additional Review Considerations
Budget: The reasonableness of the proposed budget and the requested period of
support in relation to the proposed research. The priority score should not be
affected by the evaluation of the budget.
Applications from Foreign Organizations: Does the project presents special opportunities for furthering research programs through the use of unusual talent, resources, populations, or environmental conditions in other countries that are not readily available in the United States or that augment existing U.S. resources will be assessed.�
Resubmission Applications
(formerly �revised/amended� applications): In addition to the above
criteria, the following criteria will be applied to resubmission applications:�
Are the responses to comments from the previous scientific review group adequate?
Are the improvements in the resubmission application appropriate?
2.C.
Sharing Research Data
The reasonableness of the data sharing plan or the
rationale for not sharing research data may be assessed by the reviewers.
However, reviewers will not factor the proposed data sharing plan into the
determination of scientific merit or the priority score. The funding
organization will be responsible for monitoring the data sharing policy (https://grants.nih.gov/grants/policy/data_sharing).
2.D. Sharing Research
Resources
NIH policy requires
that grant awardee recipients make unique research resources readily available
for research purposes to qualified individuals within the scientific community
after publication (See the NIH Grants Policy Statement� https://grants.nih.gov/archive/grants/policy/nihgps_2003/index.htm#_Toc54600131).
Investigators responding to this funding opportunity should include a sharing
research resources plan addressing how unique research resources will be shared
or explain why sharing is not possible.
Program staff will be responsible for the
administrative review of the plan for sharing research resources.
The adequacy of the resources
sharing plan and any related data sharing plans will be considered by Program
staff of the funding organization when making recommendations about funding
applications. The effectiveness of the resource sharing will be evaluated as
part of the administrative review of each Non-Competing Grant
Progress Report (PHS 2590), See Section VI.3.,
�Reporting.�
Model Organism
Sharing Plan: Reviewers are
asked to assess the sharing plan in an administrative note. The sharing plan
itself should be discussed after the application is scored. Whether a sharing
plan is reasonable can be determined by the reviewers on a case-by-case basis,
taking into consideration the organism, the timeline, the applicant's decision
to distribute the resource or deposit it in a repository, and other relevant
considerations
3. Anticipated Announcement and Award Dates
Not Applicable
Section VI. Award Administration Information
1.
Award Notices
After the peer review of the application is completed, the PD/PI will be able
to access his/her Summary Statement (written critique) via the NIH eRA Commons.
If the
application is under consideration for funding, NIH will request
"just-in-time" information from the applicant. For details,
applicants may refer to the NIH
Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards,
Subpart A: General.
A formal notification in the form of a Notice of Award
(NoA) will be provided to the applicant organization. The NoA signed by the
grants management officer is the authorizing document. Once all administrative
and programmatic issues have been resolved, the NoA will be generated via email
notification from the awarding component to the grantee business official.
Selection of an application for award is not an
authorization to begin performance. Any costs incurred before receipt of the
NoA are at the recipient's risk. These costs may be reimbursed only to the
extent considered allowable pre-award costs. See Section IV.5., �Funding
Restrictions.�
2.
Administrative and National Policy Requirements
There will be terms and
conditions on awards pertaining to data and resources release policies (where
applicable) that have been agreed upon by the applicants and the program staff.
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Grantees, and Activities.
When multiple years are involved, awardees will be required to submit the Non-Competing Grant Progress Report (PHS 2590) annually and financial statements as required in the NIH Grants Policy Statement.
We
encourage your inquiries concerning this funding opportunity and welcome the
opportunity to answer questions from potential applicants. Inquiries may fall
into three areas: scientific/research, peer review, and financial or grants
management issues:
1. Scientific/Research
Contacts:
Patricia L.
Mabry, Ph.D.
Health Scientist
Administrator/Behavioral Scientist
Office of Behavioral and Social Science Research
Office of the Director, NIH
31 Center Drive, Building 31, Room B2B37; MSC 2027
Bethesda, MD 20892-2027
Phone: (301) 402-1753
FAX: (301) 402-1150
Email: [email protected]
Lisa Onken, Ph.D.
Chief, Behavioral and Integrative
Treatment Branch
Division of Clinical Neuroscience and Behavioral Research
National Institute on Drug Abuse, NIH
6001 Executive Boulevard, Room 3172 MSC 9593
Bethesda, MD 20892-9593
Telephone: (301) 443-2235
FAX: (301) 443-6814
Email: [email protected]
2. Peer Review Contacts:
Teresa Levitin,
Ph.D.
Office
of Extramural Affairs
National
Institute on Drug Abuse, NIH, DHHS
6101
Executive Boulevard, Suite 234, MSC 8401
Bethesda, Maryland� 20892-8401
Telephone:�
(301) 443-2755
FAX:�
(301) 443-0538
Email:� [email protected]
3. Financial or Grants
Management Contacts:
Deborah S. Wertz
Grants Management Branch
National Institute on Drug
Abuse/NIH/DHHS
6101 Executive Boulevard, Room 270
Bethesda, MD 20892-8403
Telephone: (301) 443-6710
FAX: (301) 594-6849
Email: [email protected]
Section VIII. Other Information
Required Federal Citations
Use of Animals in Research:
Recipients of PHS support for activities involving
live, vertebrate animals must comply with PHS Policy on Humane Care and Use of Laboratory
Animals (https://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf)
as mandated by the Health Research Extension Act of 1985 (https://grants.nih.gov/grants/olaw/references/hrea1985.htm),
and the USDA Animal Welfare Regulations (http://www.nal.usda.gov/awic/legislat/usdaleg1.htm)
as applicable.
Human Subjects Protection:
Federal regulations (45 CFR 46) require that
applications and proposals involving human subjects must be evaluated with
reference to the risks to the subjects, the adequacy of protection against these
risks, the potential benefits of the research to the subjects and others, and
the importance of the knowledge gained or to be gained (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm).
Data and Safety Monitoring Plan:
Data and safety monitoring is required for all types
of clinical trials, including physiologic toxicity and dose-finding studies
(phase I); efficacy studies (Phase II); efficacy, effectiveness and comparative
trials (Phase III). Monitoring should be commensurate with risk. The
establishment of data and safety monitoring boards (DSMBs) is required for
multi-site clinical trials involving interventions that entail potential risks
to the participants (�NIH Policy for Data and Safety Monitoring,� NIH Guide
for Grants and Contracts, https://grants.nih.gov/grants/guide/notice-files/not98-084.html).
Sharing Research Data:
Investigators submitting an NIH application seeking
$500,000 or more in direct costs in any single year are expected to include a
plan for data sharing or state why this is not possible (https://grants.nih.gov/grants/policy/data_sharing).
Investigators should seek guidance from their
institutions, on issues related to institutional policies and local IRB rules,
as well as local, State and Federal laws and regulations, including the Privacy
Rule. Reviewers will consider the data sharing plan but will not factor the
plan into the determination of the scientific merit or the priority score.
Access to Research Data through
the Freedom of Information Act:
The Office of Management and Budget (OMB) Circular A-110
has been revised to provide access to research data through the Freedom of
Information Act (FOIA) under some circumstances. Data that are (1) first
produced in a project that is supported in whole or in part with Federal funds
and (2) cited publicly and officially by a Federal agency in support of an
action that has the force and effect of law (i.e., a regulation) may be
accessed through FOIA. It is important for applicants to understand the basic
scope of this amendment. NIH has provided guidance at https://grants.nih.gov/grants/policy/a110/a110_guidance_dec1999.htm.
Applicants may wish to place data collected under this funding opportunity in a
public archive, which can provide protections for the data and manage the
distribution for an indefinite period of time. If so, the application should
include a description of the archiving plan in the study design and include
information about this in the budget justification section of the application.
In addition, applicants should think about how to structure informed consent
statements and other human subjects procedures given the potential for wider
use of data collected under this award.
Sharing of Model Organisms:
NIH is committed to support efforts that encourage
sharing of important research resources including the sharing of model
organisms for biomedical research (see https://grants.nih.gov/grants/policy/model_organism/index.htm).
At the same time the NIH recognizes the rights of grantees and contractors to
elect and retain title to subject inventions developed with Federal funding
pursuant to the Bayh Dole Act (see the NIH Grants Policy Statement.
Beginning October 1, 2004, all investigators submitting an NIH application or
contract proposal are expected to include in the application/proposal a
description of a specific plan for sharing and distributing unique model
organism research resources generated using NIH funding or state why such
sharing is restricted or not possible. This will permit other researchers to
benefit from the resources developed with public funding. The inclusion of a
model organism sharing plan is not subject to a cost threshold in any year and
is expected to be included in all applications where the development of model
organisms is anticipated.
Inclusion of Women And Minorities in Clinical
Research:
It is the policy of the NIH that women and members of
minority groups and their sub-populations must be included in all NIH-supported
clinical research projects unless a clear and compelling justification is
provided indicating that inclusion is inappropriate with respect to the health
of the subjects or the purpose of the research. This policy results from the
NIH Revitalization Act of 1993 (Section 492B of Public Law 103-43). All
investigators proposing clinical research should read the "NIH Guidelines
for Inclusion of Women and Minorities as Subjects in Clinical Research� (https://grants.nih.gov/grants/guide/notice-files/NOT-OD-02-001.html);
a complete copy of the updated Guidelines is available at https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm.
The amended policy incorporates: the use of an NIH definition of clinical
research; updated racial and ethnic categories in compliance with the new OMB
standards; clarification of language governing NIH-defined Phase III clinical
trials consistent with the SF424 (R&R) application; and updated roles and responsibilities
of NIH staff and the extramural community. The policy continues to require for
all NIH-defined Phase III clinical trials that: a) all applications or
proposals and/or protocols must provide a description of plans to conduct
analyses, as appropriate, to address differences by sex/gender and/or
racial/ethnic groups, including subgroups if applicable; and b) investigators
must report annual accrual and progress in conducting analyses, as appropriate,
by sex/gender and/or racial/ethnic group differences.
Inclusion of Children as Participants in Clinical
Research:
The NIH maintains a policy that children (i.e.,
individuals under the age of 21) must be included in all clinical research,
conducted or supported by the NIH, unless there are scientific and ethical
reasons not to include them.
All investigators proposing research involving human
subjects should read the "NIH Policy and Guidelines" on the inclusion
of children as participants in research involving human subjects (https://grants.nih.gov/grants/funding/children/children.htm).
Required Education on the Protection of Human
Subject Participants:
NIH policy requires education on the protection of
human subject participants for all investigators submitting NIH applications
for research involving human subjects and individuals designated as key
personnel. The policy is available at https://grants.nih.gov/grants/guide/notice-files/NOT-OD-00-039.html.
Human Embryonic Stem Cells (hESC):
Criteria for federal funding of research on hESCs can
be found at http://stemcells.nih.gov/index.asp and at https://grants.nih.gov/grants/guide/notice-files/NOT-OD-02-005.html.
Only research using hESC lines that are registered in the NIH Human Embryonic
Stem Cell Registry will be eligible for Federal funding (http://escr.nih.gov). It is the responsibility
of the applicant to provide in the project description and elsewhere in the
application as appropriate, the official NIH identifier(s) for the hESC line(s)
to be used in the proposed research. Applications that do not provide this
information will be returned without review.
NIH Public Access Policy:
NIH-funded investigators are requested to submit to
the NIH manuscript submission (NIHMS) system (http://www.nihms.nih.gov) at
PubMed Central (PMC) an electronic version of the author's final manuscript
upon acceptance for publication, resulting from research supported in whole or
in part with direct costs from NIH. The author's final manuscript is defined as
the final version accepted for journal publication, and includes all
modifications from the publishing peer review process.
NIH is requesting that authors submit manuscripts
resulting from 1) currently funded NIH research projects or 2) previously
supported NIH research projects if they are accepted for publication on or
after May 2, 2005. The NIH Public Access Policy applies to all research grant
and career development award mechanisms, cooperative agreements, contracts,
Institutional and Individual Ruth L. Kirschstein National Research Service
Awards, as well as NIH intramural research studies. The Policy applies to
peer-reviewed, original research publications that have been supported in whole
or in part with direct costs from NIH, but it does not apply to book chapters,
editorials, reviews, or conference proceedings. Publications resulting from
non-NIH-supported research projects should not be submitted.
For more information about the Policy or the submission
process, please visit the NIH Public Access Policy Web site at http://publicaccess.nih.gov/
and view the Policy or other Resources and Tools, including the Authors' Manual.
Standards for Privacy of Individually Identifiable
Health Information:
The Department of Health and Human Services (HHS)
issued final modification to the "Standards for Privacy of Individually
Identifiable Health Information", the "Privacy Rule", on August 14, 2002. The Privacy Rule is a federal regulation under the Health Insurance
Portability and Accountability Act (HIPAA) of 1996 that governs the protection
of individually identifiable health information, and is administered and
enforced by the HHS Office for Civil Rights (OCR).
Decisions about applicability and implementation of
the Privacy Rule reside with the researcher and his/her institution. The OCR
website (http://www.hhs.gov/ocr/)
provides information on the Privacy Rule, including a complete Regulation Text
and a set of decision tools on "Am I a covered entity?" Information
on the impact of the HIPAA Privacy Rule on NIH processes involving the review,
funding, and progress monitoring of grants, cooperative agreements, and
research contracts can be found at https://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-025.html.
URLs in NIH Grant Applications or Appendices:
All applications and proposals for NIH funding
must be self-contained within specified page limitations. For publications
listed in the appendix and/or Progress report, internet addresses (URLs) must be used for publicly accessible on-line journal articles.� Unless
otherwise specified in this solicitation, Internet addresses (URLs)
should not be used to provide any other information necessary for
the review because reviewers are under no obligation to view the Internet
sites. Furthermore, we caution reviewers that their anonymity may be
compromised when they directly access an Internet site.
Healthy
People 2010:
The Public Health
Service (PHS) is committed to achieving the health promotion and disease
prevention objectives of "Healthy People 2010," a PHS-led national
activity for setting priority areas. This FOA is related to one or more of the
priority areas. Potential applicants may obtain a copy of "Healthy People
2010" at http://www.health.gov/healthypeople.
Authority and
Regulations:
This
program is described in the Catalog of Federal Domestic Assistance at http://www.cfda.gov/ and is not subject to the
intergovernmental review requirements of Executive
Order 12372 or Health Systems Agency review. Awards are made under the
authorization of Sections 301 and 405 of the Public Health Service Act as
amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and
45 CFR Parts 74 and 92. All awards are subject to the
terms and conditions, cost principles, and other considerations described in
the NIH Grants
Policy Statement.
The PHS strongly encourages
all grant recipients to provide a smoke-free workplace and discourage the use
of all tobacco products. In addition, Public Law 103-227, the Pro-Children Act
of 1994, prohibits smoking in certain facilities (or in some cases, any portion
of a facility) in which regular or routine education, library, day care, health
care, or early childhood development services are provided to children. This is
consistent with the PHS mission to protect and advance the physical and mental
health of the American people.
Loan Repayment
Programs:
NIH encourages
applications for educational loan repayment from qualified health professionals
who have made a commitment to pursue a research career involving clinical,
pediatric, contraception, infertility, and health disparities related areas.
The LRP is an important component of NIH's efforts to recruit and retain the
next generation of researchers by providing the means for developing a research
career unfettered by the burden of student loan debt. Note that an NIH grant is
not required for eligibility and concurrent career award and LRP applications
are encouraged. The periods of career award and LRP award may overlap providing
the LRP recipient with the required commitment of time and effort, as LRP
awardees must commit at least 50% of their time (at least 20 hours per week
based on a 40 hour week) for two years to the research. For further
information, please see: http://www.lrp.nih.gov.