It is critical that applicants follow the Research (R) Instructions in the SF424 (R&R) Application Guide, except where instructed to do otherwise (in this FOA or in a Notice from the NIH Guide for Grants and Contracts). Conformance to all requirements (both in the Application Guide and the FOA) is required and strictly enforced. Applicants must read and follow all application instructions in the Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the Application Guide, follow the program-specific instructions. Applications that do not comply with these instructions may be delayed or not accepted for review.
Part 1. Overview Information
Part 2. Full Text of the Announcement
Section
I. Funding Opportunity Description
Section II. Award Information
Section III. Eligibility Information
Section IV. Application and Submission
Information
Section V. Application Review Information
Section VI. Award Administration Information
Section VII. Agency Contacts
Section VIII. Other Information
The Food and Drug Administration (FDA), Office of Regulatory Affairs (ORA) is announcing the availability of a cooperative agreement to be awarded under Limited Competition to a national association or organization whose membership includes State, local, territorial, and/or tribal (SLTT) retail food safety regulators or SLTT government agencies that are responsible for retail food safety.
The intended purpose of this FOA is to advance conformance with the Voluntary National Retail Food Regulatory Program Standards (VNRFRPS). Funds will be awarded to a national association/organization to assist State, local, territorial, and tribal (SLTT) jurisdictions to conform with the VNRFRPS by achieving the objectives described below.
1. Develop and implement a system to administer financial assistance to State, local, territorial, and tribal retail regulatory food programs based on the FDA's VNRFRPS flexible funding model.
2. Develop and implement a standardized method to assess training needs of retail food regulatory jurisdictions and facilitate meeting those needs
3. Develop and implement a tracking system that quantifies the extent of Standardization of regulatory food safety inspection personnel within, and among, regulatory retail food jurisdictions.
Financial assistance to SLTTs, defined under objective one, should utilize a Flexible Funding Model established in collaboration with the grantee and FDA. A suggested basic model is listed below:
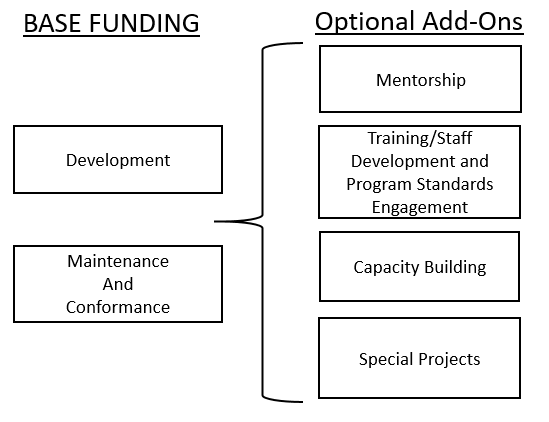
Base funding is a list of required activities to receive funding from FDA. The optional add-on categories can be offered to those receiving funding in the base of the model.
Once the cooperative agreement has been awarded, FDA will work with the grantee on the expected eligibility requirements, financial assistance rates, and other aspects of any financial assistance offered to SLTTs.
Any funding system developed by the grantee to provide financial assistance to SLTTs should meet the following criteria:
- Establishes a process for applicants to apply for funding based on the VNRFRPS flexible funding model.
- Establishes a system for collection of performance outcomes to document return on investment of the sub-awarded funds.
- Establishes a system for monitoring the progress of sub-awardees to include the periodic verification of project milestones, performance outcomes, and reporting of the project results to FDA.
- Establishes a system for the management, distribution, and verification of the use of funds sub-awarded to retail food regulatory programs.
- Establishes a system for development of corrective action plans when sub-awardees are not completing the projects proposed or using the funds appropriately and notify the FDA Project Manager of the related issue(s).
- Contains a communications strategy that includes advertising the availability of funds, projects funded, and project results.
- Describes unbiased methods for solicitation of applications, review of submissions, and selection of sub-awardees to ensure distribution of funds over a wide range of State, local, tribal, and territorial retail food regulatory jurisdictions in keeping with FDA program priorities.
- Involves a joint advisory group including the grantee, FDA, and other stakeholders to establish priorities and to ensure the goals of the cooperative agreement are achieved.
- Will ensure that the projects proposed by sub-awardees have not been reimbursed under other cooperative agreements, grants, contracts, and other funding mechanisms.
Projects proposed by the sub-awardees should target the requirements of the approved VNRFRPS flexible funding model. Programs applying for sub-awards should submit a proposal detailing the use and outcomes of the funds requested and a progress report at the conclusion of any funded project.
For Objective #1, the data collected should track the following performance outcomes based on the type of funding the SLTT has been approved to use:
1. Completion of a self-assessment of the enrolled jurisdiction's regulatory retail program against all nine VNRFRPS Standards.
2. Development and implementation of a viable action plan to address gaps identified in the SLTT's current self-assessment.
3. Increased conformance with meeting the Standards criteria during the current self-assessment period.
4. Completion of a retail food risk factor study or an equivalent public health metric.
5. Implementation of an intervention strategy or strategies designed to reduce the occurrence of foodborne illness risk factors.
6. Completion of verification audit(s) of Standards reported as being met during their current self-assessment period.
7. Conformance with all nine standards, confirmed by a verification audit, during the current self-assessment period.
For Objective #2, the data collected should track the following performance outcomes:
- Creating a report that summarizes the development of the parameters for assessing training needs.
- Creating a report that summarizes the protocol for assessing training needs and the findings of the assessment.
- Creating a report that describes the method for facilitating registration for retail food protection training.
For Objective #3, the data collected should track the following performance outcome:
Creating a report that summarizes the key findings from the tracking system related to Objective 3, including but not limited to the number of Standardization(s) performed, the types of Standardizations performed, and the number of Standardized persons currently meeting the maintenance requirements.
The cooperative agreement includes one year of funding with up to two years of additional non-competitive support, dependent on grantee performance and continued availability of federal funds.
The VNRFRPS apply to the operation and management of a retail food regulatory program
by providing a framework designed to accommodate both traditional and emerging approaches to food safety with a focus on reducing the occurrence of foodborne illness risk factors. The VNRFRPS include nine individual Program Standards. Each Program Standard has one or more corresponding worksheet(s), forms and explanatory documents. While the VNRFRPS represent a model of an effective, focused retail food protection program, they begin by providing foundational criteria upon which all regulatory programs can assess and build effective infrastructures through a continuous improvement process.
This funding opportunity furthers FDA's efforts to enhance state, local, territorial, and tribal (SLTT) retail food protection programs. Legislative and strategic initiatives have addressed FDA's relationship with SLTT authorities in retail food protection activities.
a) Food and Drug Administration Amendments Act of 2007 (FDAAA)
Under FDAAA, FDA is required to work with the states to improve food safety. Section 1004 of FDAAA states:
SEC. 1004. STATE AND FEDERAL COOPERATION
(a) IN GENERAL. The Secretary shall work with the states in undertaking activities and programs that assist in improving the safety of food, including fresh and processed produce, so that state food safety programs and activities conducted by the Secretary function in a coordinated and cost-effective manner. With the assistance provided under subsection (b), the Secretary shall encourage states to
(1) Establish, continue, or strengthen state food safety programs, especially with respect to the regulation of retail commercial food establishments; and (2) Establish procedures and requirements for ensuring that processed produce under the jurisdiction of state
food safety programs is not unsafe for human consumption.
(b) ASSISTANCE. The Secretary may provide to a state, for planning, developing, and implementing such a food safety program
(1) Advisory assistance;
(2) Technical assistance, training, and laboratory assistance (including necessary materials and equipment); and
(3) Financial and other assistance.
(c) SERVICE AGREEMENTS. The Secretary may, under an agreement entered into with a federal, state, or local agency, use, on a reimbursable basis or otherwise, the personnel, services, and facilities of the agency to carry out the responsibilities of the agency under this section. An agreement entered into with a state agency under this subsection may provide for training of state employees.
b) FDA Food Safety Modernization Act
The FDA Food Safety Modernization Act (FSMA), signed into law on January 4, 2011, provides FDA with tools to better protect public health by strengthening the food safety system. It enables FDA to focus on preventing food safety problems rather than reacting to problems after they occur. It also provides FDA with new enforcement authorities designed to achieve higher rates of compliance with prevention- and risk-based food safety standards and to better respond to and contain problems when they do occur. These include authorities such as mandatory recall, expanded administrative detention, suspension of facility registration, enhanced product tracing abilities, and additional recordkeeping requirements for high-risk foods. FSMA also gives FDA important new tools to hold imported foods to the same standards as domestic foods. FSMA directs FDA to build an integrated national food safety system in partnership with state and local authorities, explicitly recognizing that all food safety agencies need to work together in an integrated way to achieve national public health goals. FSMA identifies some key priorities in working with partners in areas such as: reliance on federal, state, and local agencies for inspections; improving foodborne illness surveillance; and leveraging and enhancing State and Local food safety and defense capacities
c) Retail Food Safety Initiative
Announced in October 2010, the Retail Food Safety Initiative is part of the Food and Drug Administration’s overall prevention-based, farm-to-table food safety strategy to reduce foodborne illness. The FDA actions in this initiative are prompted by a 10-year study of more than 800 retail food establishments to determine compliance with five key risk factors in nine types of retail operations.
FDA's partnerships with the retail and foodservice industries; state, local, territorial, and tribal regulatory authorities; and other government agencies are a foundational building block of the initiative and key to its success in four action areas:
-Make the presence of certified food protection managers a common practice.
-Strengthen active managerial controls at the retail level and ensure better compliance.
-Encourage widespread, uniform, and complete adoption of the FDA Food Code.
-Create an enhanced local regulatory environment for retail food operations by:
(1) Promoting wider implementation by state, local and tribal regulatory programs of the FDA Voluntary National Retail Food Regulatory Programs Standards.
(2) Ensuring universal participation by local regulators in consistent, high quality training through increased access and increased portability and transferability of FDA courses.
(3) Seeking increased support for the state, local and tribal programs as part of an integrated food safety system.
d) New Era for Smarter Food Safety
On April 30, 2019, the FDA announced a new approach to food safety, one that recognizes and builds on the progress made in the past but looks towards what processes and tools will be needed for the future. This work will build on the advances that have been and are being made in FDA s implementation of FSMA while advancing the use of technologies that are currently used in society and business sectors all around us. The VNRFRPS provides a foundational framework for an integrated food safety system for regulatory retail food protection programs.
For more information: https://www.fda.gov/food/food-industry/new-era-smarter-food-safety
e) Healthy People 2030
Healthy People is a national effort that sets goals and objectives to improve the health and well-being of people in the United Sates.
Healthy People established foundational principles, overarching goals, and a plan of action to promote, strengthen and evaluate the Nation's efforts to improve the health and well-being of all people.
Healthy People 2030 is the fifth edition of Healthy People. It aims at new challenges and builds on lessons learned from its first 4 decades. The initiative began in 1979, when Surgeon General Julius Richmond issued a landmark report entitled, Healthy People: The Surgeon General s Report on Health Promotion and Disease Prevention. This report focused on reducing preventable death and injury. It included ambitious, quantifiable objectives to achieve national health promotion and disease prevention goals for the United States within a 10-year period (by 1990). The report was followed in later decades by the release of updated, 10-year Healthy People goals and objectives (Healthy People 2000, Healthy People 2010, and Healthy People 2020).
For more information: https://www.healthypeople.gov/
See Section VIII. Other Information for award authorities and regulations.
HHS grants policies as described in the HHS Grants Policy Statement will apply to the applications submitted and awards made from this FOA.
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
This opportunity is only available to the following nonprofit food safety training entities that collaborate with 1 or more institutions of higher education:
National organization/associations that perform food safety training and whose membership includes, but is not necessarily limited to, state, local, territorial, and/or tribal regulatory retail food safety professionals or government agencies and who collaborate with one or more institutions of higher education.
Competition is limited to these national organizations/associations for the following reasons:
1. National organizations/associations with membership that includes, but is not necessarily limited to, SLTT regulatory retail food professionals or government agencies will have the relationships and communication systems to effectively promote the objectives established under this cooperative agreement.
2. These national organizations/associations have a vested interest and share FDA’s visions to advance the VNRFRPS and improve public health outcomes. They are also knowledgeable of the VNRFRPS and the requirements to achieve conformance. These national organizations/associations can best collaborate with FDA.
3. These national organizations/associations can provide a platform for sharing information and national implementation of the projects pursued by the subawardees through national and regional meetings, web sites, listservs, and other communications. Most national associations/organizations also have committees that may further advance the projects pursued by the subawardees.
Non-domestic (non-U.S.) Entities (Foreign Institutions) are
not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible
to apply.
Foreign components, as defined in the HHS
Grants Policy Statement, are not allowed.
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the SF 424 (R&R) Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission.
- Dun and Bradstreet Universal Numbering System (DUNS) - All registrations require that applicants be issued a DUNS number. After obtaining a DUNS number, applicants can begin both SAM and eRA Commons registrations. The same DUNS number must be used for all registrations, as well as on the grant application.
- System for Award Management (SAM) Applicants must complete and maintain an active registration, which requires renewal at least annually. The renewal process may require as much time as the initial registration. SAM registration includes the assignment of a Commercial and Government Entity (CAGE) Code for domestic organizations which have not already been assigned a CAGE Code.
- NATO Commercial and Government Entity (NCAGE) Code Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- eRA Commons - Applicants must have an active DUNS number to register in eRA Commons. Organizations can register with the eRA Commons as they are working through their SAM or Grants.gov registration, but all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov Applicants must have an active DUNS number and SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with his/her organization to develop an application for support. Individuals from underrepresented racial and ethnic groups as well as individuals with disabilities are always encouraged to apply for FDA support.
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the SF424 (R&R) Application Guide.
This FOA does not require cost sharing as defined in the HHS Grants Policy Statement.
Applicant organizations may submit more than one application, provided that each application is scientifically distinct.
The FDA will not accept duplicate or highly overlapping applications under review at the same time. This means that the FDA will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
The application forms package specific to this opportunity must be accessed through ASSIST, Grants.gov Workspace or an institutional system-to-system solution. Links to apply using ASSIST or Grants.gov Workspace are available in Part 1 of this FOA. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
It is critical that applicants follow the Research (R) Instructions in the SF424 (R&R) Application Guide, except where instructed in this funding opportunity announcement to do otherwise. Conformance to the requirements in the Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows FDA staff to estimate the potential review workload and plan the review.
By the date listed in Part 1. Overview Information, prospective applicants are asked to submit a letter of intent that includes the following information:
- Descriptive title of proposed activity
- Name(s), email address(es), and telephone number(s) of the PD(s)/PI(s)
- Names of other key personnel
- Participating institution(s)
- Number and title of this funding opportunity
The letter of intent should be sent to:
Gordana Zuber
Telephone: 301-348-1747
Email: gordana.zuber@fda.hhs.gov
A technical session will be held for prospective applicants in February 2021. The conference call information will be provided to prospective applicants that submit a letter of intent. The technical session will provide an overview of the submission requirements and allow prospective applicants an opportunity to ask questions regarding the application process. Participation in the technical session is optional, but strongly encouraged.
All page limitations described in the SF424 Application Guide and the Table of Page Limits must be followed, with the following exceptions or additional requirements:
For this specific FOA, the Research Strategy section is limited to 30 pages.
The following section supplements the instructions found in the SF424 (R&R) Application Guide and should be used for preparing an application to this FOA.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions:
- Applications requesting multiple years of support must complete and submit a separate detailed budget breakdown and narrative justification for each year of financial support requested.
- Applicants must provide a properly detailed budget (one for each of the three years) that is intended to complete defined objectives and award and administer funds for the advancement of the VNRFRPS.
- If an applicant is requesting indirect costs as part of their budget, a copy of the most recent Federal indirect cost rate or F&A agreement must be provided as part of the application submission. This agreement should be attached to the RESEARCH & RELATED Other Project Information Component as line #12 'Other Attachments'.
- If the applicant organization has never established an indirect cost rate and/or does not have a negotiated Federal indirect cost rate agreement, a de minimis indirect cost rate of 10 percent (10%) of modified total direct costs (MTDC) will be allowed. MTDC means all direct salaries and wages, applicable fringe benefits, materials and supplies, services, travel, and subaward and subcontracts up to the first $25,000 of each subaward or subcontract. MTDC excludes equipment, capital expenditures, charges for patient care, rental costs, tuition remission, scholarships and fellowships, participant support costs and the portion of each subaward and subcontract in excess of $25,000.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed.
All instructions in the SF424 (R&R) Application Guide must be followed, with the following additional instructions:
Research Strategy: In the Research Strategy, the applicant shall specifically address the ability to perform the following activities to achieve the objectives in the cooperative agreement:
1. Demonstrate the ability to develop and implement a comprehensive strategic plan that includes goals and project milestones that will result in meeting the objectives of the funding announcement including the development, implementation, and management of a program to financially assist regulatory retail food protection programs. The plan should include information related to the data systems that will be developed and used to meet the objectives.
2. Demonstrate the ability to engage and collaborate with regulatory retail food protection programs, FDA, and other stakeholders to meet the goals and objectives of this cooperative agreement and proposed project.
3. Demonstrate ability to establish an on-line portal/data management system to manage all aspects of providing financial assistance.
4. Demonstrate the availability of adequately trained staff and, if needed, the ability to hire and/or train personnel to meet the deliverables of the cooperative agreement. Provide justification for hiring new staff, including qualifications, training needs, and new equipment needs.
5. Demonstrate the ability to satisfy the reporting requirements outlined in this announcement.
6. Outline a detailed methodology for program assessment, improvement, and collaboration to accomplish the work, as described in this announcement. Program assessment will include predefined performance outcomes required by FDA.
6. Demonstrate the ability to provide administrative oversight for funds awarded to regulatory retail food protection programs (sub-awardees) and/or similar associations through this cooperative agreement, including distribution of funds, monitoring project deliverables and expenditures, and implementing corrective actions when necessary.
Additionally, the applicant must submit as part of the Appendix:
(A) an assurance that the eligible entity has developed plans to engage in the types of activities described in this FOA. This assurance must be uploaded as Appendix #1.
(B) an agreement by the eligible entity to report information required by the Secretary to conduct evaluations under this section. This agreement must be uploaded as Appendix 2.
Resource Sharing Plan: Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the SF424 (R&R) Application Guide, with the following modification:
- Generally, Resource Sharing Plans are expected, but they are not applicable for this FOA.
Appendix:
Only limited Appendix materials are allowed. Follow all instructions for the Appendix as described in the SF424 (R&R) Application Guide, with the following additional requirements:
(A) an assurance that the eligible entity has developed plans to engage in the types of activities described in this FOA. This assurance must be uploaded as Appendix #1.
(B) an agreement by the eligible entity to report information required by the Secretary to conduct evaluations under this section. This agreement must be uploaded as Appendix 2.
All instructions in the SF424 (R&R) Application Guide must be followed.
See Part 1. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
Part I. Overview Information contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies). Applicants must then complete the submission process by tracking the status of the application in the eRA Commons, FDA’s electronic system for grants administration. eRA Commons and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Late applications will not be accepted for this FOA.
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the SF424 (R&R) Application Guide.
This initiative is not subject to intergovernmental review.
All FDA awards are subject to the terms and conditions, cost principles, and other considerations described in the HHS Grants Policy Statement.
Pre-award costs are allowable only as described in the HHS Grants Policy Statement.
A majority of the cooperative agreement funds should be spent to build relationships and assist SLTT regulatory agencies with primary responsibility for retail food establishments in their jurisdictions or to agencies with substantial control and responsibility over such agencies, which may include financial support.
If another national association or organization is utilized by the grantee to administer portions of the objectives, any associated fees must be allocated from the grantee's budget.
In addition, work proposed and conducted under this cooperative agreement, including subawards issued, may not be duplicated or funded by other cooperative agreements, contracts, or other funding mechanisms. Projects proposed and conducted under this cooperative agreement and the funding provided shall remain distinct and separate from other projects and funding sources. The grantee shall be able to account separately for fund expenditures, including employee salaries, wages, and benefits, received through contracts, cooperative agreements, grants, and other funding received by the grantee and these cooperative agreements.
Non-allowable costs:
1. Facilities, work, training, and other expenses reimbursed under other cooperative agreements, grants, contracts, and other funding mechanisms shall remain distinct and separate from this cooperative agreement.
2. Vehicle purchases are not permitted.
3. Cooperative agreement may not be utilized for new building construction or remodeling of existing facilities.
Additional funding restrictions may be part of the Notice of Award.
Applications must be submitted electronically following the instructions described in the SF424 (R&R) Application Guide. Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply Application Guide. For assistance with application submission, contact the Application Submission Contacts in Section VII.
Important reminders:
All PD(s)/PI(s) must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile Component of the SF424(R&R) Application Package. Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to FDA. See Section III of this FOA for information on registration requirements.
The applicant organization must ensure that the DUNS number it provides on the application is the same number used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the SF424 (R&R) Application Guide.
See more tips for avoiding common errors.
Upon receipt, applications will be evaluated for completeness and compliance with application instructions by the assigned Grants Management Specialist and responsiveness by components of participating organizations, FDA. Applications that are incomplete, non-compliant and/or nonresponsive will not be reviewed.
Post-submission materials are those submitted after submission of the grant application but prior to objective review. They are not intended to correct oversights or errors discovered after submission of the application. FDA accepts limited information between the time of initial submission of the application and the time of objective review. Applicants must contact the assigned Grants Management Specialist to receive approval, prior to submitting any post submission materials. Acceptance and/or rejection of any post submission materials is at the sole discretion of the FDA. Any inquiries regarding post submission materials should be directed to the assigned Grants Management Specialist.
Only the review criteria described below will be considered in the review process.
Reviewers will consider each of the review criteria below in the determination of scientific merit.
Significance and Approach (40 points)
Does the applicant address each of the objectives of the funding announcement?
Does the applicant demonstrate the organizational capacity to manage the project and provide an infrastructure capable of monitoring progress toward achievement of project milestones?
Does the application demonstrate the capacity and capability to implement a VNRFRPS flexible funding model and distribute, track and monitor sub-awarded funding to SLTT s?
Does organization plan to leverage their work with other organizations? If so, is there adequate demonstration of effectiveness in working with other agencies and appropriate organizations to implement the goals of the cooperative agreement?
Are the proposed system, strategies, and approach to meet the intended outcome of the cooperative agreement well-reasoned, appropriate, and complete?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project?
Established Relationships and Quality Improvement (30 points)
Does the applicant demonstrate the number and strength of relationships with retail food safety regulatory agencies and public health stakeholders necessary to ensure success of the project?
Does the applicant provide demonstration of effectiveness in working with federal, state, local, territorial, and/or tribal regulatory jurisdictions on food safety issues that positions the applicant to successfully implement the intended outcome of the cooperative agreement?
Did the applicant address the expected challenges and minimize barriers to completing the overall project?
Outreach (10 points)
Did the applicant provide a strategy to engage State, Local, Tribal and Territorial retail food protection programs ensuring wide-distribution of both funding opportunities and the projects developed to meet the objectives of the FOA?
Investigator, Key Personnel, and Environment (10 points)
Are the PD(s)/PI(s), collaborators, and other key personnel well suited to the project, including having appropriate experience and training? Has the applicant demonstrated adequate program resources including staff and technology infrastructure, or the ability to obtain the resources necessary, to sustain and complete the activities described in the FOA (including the ability to process and administer subawards)?
Information Technology Development (10 points)
Does the proposal outline a sufficiently robust information technology solution to manage data
generated by the program and demonstrate public health impact?
As applicable for the project proposed, reviewers will evaluate the following additional items but will not give separate scores for these items, and should not consider them in providing an overall score.
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Inclusion of Women, Minorities, and Individuals Across the Lifespan
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Not Applicable.
Not Applicable.
Not Applicable.
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Generally not applicable. Reviewers should bring any concerns to the attention of assigned Grants Management Specialist.
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
Applications will be evaluated for scientific and technical merit by (an) appropriate Objective Review Committee, using the stated review criteria.
As part of the objective review, all applications:
- Will receive a written critique.
Appeals of objective review will not be accepted for applications submitted in response to this FOA.
Applications will compete for available funds with all other recommended applications submitted in response to this FOA. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by objective review.
- Availability of funds.
- Relevance of the proposed project to program priorities.
Successful applicants will be notified of additional information that may be required or other actions leading to an award. The decision not to award a grant, or to award a grant at a particular funding level, is discretionary and is not subject to appeal to any FDA or HHS official or board.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient's business official.
Awardees must comply with any funding restrictions described in Section IV.5. Funding Restrictions and in the Notice of Award. Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this FOA will be subject to terms and conditions found in the HHS Grants Policy Statement, this FOA, and Notice of Award.
All FDA grant and cooperative agreement awards include the HHS Grants Policy Statement as part of the NoA.
Recipients of federal financial assistance (FFA) from HHS must administer their programs in compliance with federal civil rights laws that prohibit discrimination on the basis of race, color, national origin, disability, age and, in some circumstances, religion, conscience, and sex. This includes ensuring programs are accessible to persons with limited English proficiency. The HHS Office for Civil Rights provides guidance on complying with civil rights laws enforced by HHS. Please see https://www.hhs.gov/civil-rights/for-providers/provider-obligations/index.html and http://www.hhs.gov/ocr/civilrights/understanding/section1557/index.html.
HHS recognizes that research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to FDA grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this FOA.
- Recipients of FFA must ensure that their programs are accessible to persons with limited English proficiency. HHS provides guidance to recipients of FFA on meeting their legal obligation to take reasonable steps to provide meaningful access to their programs by persons with limited English proficiency. Please see https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/fact-sheet-guidance/index.html and https://www.lep.gov. For further guidance on providing culturally and linguistically appropriate services, recipients should review the National Standards for Culturally and Linguistically Appropriate Services in Health and Health Care at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=2&lvlid=53.
- Recipients of FFA also have specific legal obligations for serving qualified individuals with disabilities. Please see http://www.hhs.gov/ocr/civilrights/understanding/disability/index.html.
- HHS funded health and education programs must be administered in an environment free of sexual harassment. Please see https://www.hhs.gov/civil-rights/for-individuals/sex-discrimination/index.html; https://www2.ed.gov/about/offices/list/ocr/docs/shguide.html; and https://www.eeoc.gov/eeoc/publications/upload/fs-sex.pdf. For information about FDA's commitment to supporting a safe and respectful work environment and what FDA's expectations are for institutions and the individuals supported on FDA-funded awards, please see https://www.fda.gov/science-research/about-science-research-fda/fda-sexual-harassment-policy-concerning-extramural-research.
- Recipients of FFA must also administer their programs in compliance with applicable federal religious nondiscrimination laws and applicable federal conscience protection and associated anti-discrimination laws. Collectively, these laws prohibit exclusion, adverse treatment, coercion, or other discrimination against persons or entities on the basis of their consciences, religious beliefs, or moral convictions. Please see https://www.hhs.gov/conscience/conscience-protections/index.html and https://www.hhs.gov/conscience/religious-freedom/index.html.
Please contact the HHS Office for Civil Rights for more information about obligations and prohibitions under federal civil rights laws at https://www.hhs.gov/ocr/about-us/contact-us/index.html or call 1-800-368-1019 or TDD 1-800-537-7697.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), FDA awards will be subject to the Federal Awardee Performance and Integrity Information System (FAPIIS) requirements. FAPIIS requires Federal award making officials to review and consider information about an applicant in the designated integrity and performance system (currently FAPIIS) prior to making an award. An applicant, at its option, may review information in the designated integrity and performance systems accessible through FAPIIS and comment on any information about itself that a Federal agency previously entered and is currently in FAPIIS. The Federal awarding agency will consider any comments by the applicant, in addition to other information in FAPIIS, in making a judgement about the applicant s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 45 CFR Part 75.205 Federal awarding agency review of risk posed by applicants. This provision will apply to all FDA grants and cooperative agreements.
FDA considers the sharing of research resources developed through FDA-sponsored research an important means to enhance the value and further the advancement of research. When research resources have been developed with FDA funds and the associated research findings published, those findings must be made readily available to the scientific community.
Upon acceptance for publication, scientific researchers must submit the author’s final manuscript of the peer-reviewed scientific publication resulting from research supported in whole or in part with FDA funds to the NIH National Library of Medicine's (NLM) PubMed Central (PMC). FDA defines the author's final manuscript as the final version accepted for journal publication, which includes all modifications from the publishing peer review process. The PMC archive is the designated repository for these manuscripts for use by the public, health care providers, educators, scientists, and FDA. Please see the FDA Public Access Policy.
Additional terms and conditions regarding FDA regulatory and ORA programmatic requirements may be part of the Notice of Award.
Reporting Requirements:
All FDA grants require both Financial and Performance reporting.
Financial Reporting:
A. Cash Transaction Reports
The Federal Financial Report (FFR) has a dedicated section to report Federal cash receipts and disbursements. For recipients, this information must be submitted quarterly directly to the Payment Management System (PMS) using the web-based tool. Quarterly reports are due 30 days following the end of each calendar quarter. The reporting period for this report continues to be based on the calendar quarter. Questions concerning the requirements for this quarterly financial report should be directed to the PMS.
B. Financial Expenditure Reports
A required Federal Financial Report (FFR) must be submitted annually. All annual FFRs must be submitted electronically using the Federal Financial Report (FFR) system located in the eRA Commons. This includes all initial FFRs being prepared for submission and any revised FFRs being submitted or re-submitted to FDA. Paper expenditure/FFR reports will not accepted.
Annual FFRs must be submitted for each budget period no later than 90 days after the end of the calendar quarter in which the budget period ended. The reporting period for an annual FFR will be that of the budget period for the particular grant; however, the actual submission date is based on the calendar quarter.
Performance Progress Reporting:
When multiple years (more than one budget period) are involved, awardees will be required to submit the Research Performance Progress Report (RPPR) annually as required in the Notice of Award. Annual RPPRs must be submitted using the RPPR module in eRA Commons. The annual RPPR must include a detailed budget. Annual RPPRs are due no later than 60 days prior to the start of the next budget period.
Failure to submit timely reports may affect future funding. Additional Financial and Performance Progress reports may be required for this award. Any additional reporting requirements will be listed under Section IV Special Terms and Condition of the Notice of Award.
Salary Caps:
None of the funds in this award shall be used to pay the salary of an individual at a rate in excess
of the current Executive Level II of the Federal Executive Pay Scale.
Certificates of Confidentiality 42 U.S.C. 241(d)
Awardees are responsible for complying with all requirements to protect the confidentiality of identifiable, sensitive information that is collected or used in biomedical, behavioral, clinical, or other research (including research on mental health and research on the use and effect of alcohol and other psychoactive drugs) funded wholly or in part by the Federal Government. See 42 U.S.C. 241(d). All research funded by FDA, in whole or in part, that is within the scope of these requirements is deemed to be issued a Certificate of Confidentiality through these Terms and Conditions. Certificates issued in this manner will not be issued as a separate document.
Awardees are expected to ensure that any investigator or institution not funded by FDA who receives a copy of identifiable, sensitive information protected by these requirements, understand they are also subject to the requirements of 42 U.S.C. 241(d). Awardees are also responsible for ensuring that any subrecipient that receives funds to carry out part of the FDA award involving a copy of identifiable, sensitive information protected by these requirements understand they are also subject to subsection 42 U.S.C. 241(d).
Acknowledgment of Federal Support:
When issuing statements, press releases, publications, requests for proposal, bid solicitations and other documents --such as tool-kits, resource guides, websites, and presentations (hereafter statements )--describing the projects or programs funded in whole or in part with FDA federal funds, the recipient must clearly state:
1. the percentage and dollar amount of the total costs of the program or project funded with federal money; and,
2. the percentage and dollar amount of the total costs of the project or program funded by non-governmental sources.
When issuing statements resulting from activities supported by FDA financial assistance, the recipient entity must include an acknowledgement of federal assistance using one of the following statements.
If the FDA Grant or Cooperative Agreement is NOT funded with other non-governmental sources:
This [project/publication/program/website, etc.] [is/was] supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [FAIN] totaling $XX with 100 percent funded by FDA]/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
If the FDA Grant or Cooperative Agreement IS partially funded with other nongovernmental sources:
This [project/publication/program/website, etc.] [is/was] supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [FAIN] totaling $XX with XX percentage funded by FDA/HHS and $XX amount and XX percentage funded by non-government source(s). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
The federal award total must reflect total costs (direct and indirect) for all authorized funds (including supplements and carryover) for the total competitive segment up to the time of the public statement. Any amendments by the recipient to the acknowledgement statement must be coordinated with FDA. If the recipient plans to issue a press release concerning the outcome of activities supported by FDA financial assistance, it should notify FDA in advance to allow for coordination.
Additional prior approval requirements pertaining to Acknowledgement of Federal Support, publications, press statements, etc. may be required, and if applicable, will be listed under Section IV Special Terms and Condition of the Notice of Award.
Prior Approval:
All prior approval requests must be submitted using the Prior Approval module in eRA Commons. Any requests involving budgetary issues must include a new proposed budget and a narrative justification of the requested changes. If there are any questions regarding the need or requirement for prior approval for any activity or cost, the grantee is to contact the assigned Grants Management Specialist prior to expenditure of funds.
For grant awards not covered under Expanded Authorities, Carryover and No Cost Extension (NCE) requests will require prior approval. All Carryover and NCE requests should be submitted using the Prior Approval module in eRA Commons. ****Please review the section on Expanded Authorities to determine if this award is covered/not covered under Expanded Authorities and whether prior approval is needed for carryover and no cost extension requests.****
The following activities require prior approval from FDA on all awards:
1. Change in Grantee Organization
2. Significant Rebudgeting
3. Change in Scope or Objectives
4. Deviation from Terms and Conditions of Award
5. Change in Key Personnel which includes replacement of the PD/PI or other key personnel as specified on the NoA.
6. Disengagement from the project for more than three months, or a 25 percent reduction in time devoted to the project, by the approved PD/PI. No individual may be committed to more than 100% professional time and effort. In the event that an individual's commitment exceeds 100%, the grantee must make adjustments to reduce effort. For FDA-sponsored projects, significant reductions in effort (i.e., in excess of 25% of the originally proposed level of effort) for the PD/PI and key personnel named on named on this Notice of Award must receive written prior approval from FDA.
Additional prior approval requirements may be required for this award, and if applicable, will be listed under Section IV Special Terms and Condition of the Notice of Award.
Audits and Monitoring:
Audit Requirements:
1. Recipients of Federal funds are subject to annual audit requirements as specified in 45 CFR 75.501 (https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=8040c4036b962cc9d75c3638dedce240&ty=HTML&h=L&r=PART&n=pt45.1.75#se45.1.75_1501). Grantees should refer to this regulation for the current annual Federal fund expenditure threshold level which requires audit.
2. Foreign recipients are subject to the same audit requirements as for-profit organizations (specified in 45 CFR 75.501(h) through 75.501(k).
3. For-profit and foreign entities can email their audit reports to AuditResolution@hhs.gov or mail them to the following address:
U.S. Department of Health and Human Services
Audit Resolution Division, Room 549D
Attention: Robin Aldridge, Director
200 Independence Avenue, SW
Washington, DC 20201
Monitoring:
Recipients are responsible for managing the day-to-day operations of grant-supported activities using their established controls and policies, as long as they are consistent with Federal, DHHS and FDA requirements. However, to fulfill their role in regard to the stewardship of Federal funds, FDA monitors our grants to identify potential problems and areas where technical assistance might be necessary. This active monitoring is accomplished through review of reports and correspondence from the recipient, audit reports, site visits, and other information available to FDA.
1. Desk review: FDA grants monitoring specialists will periodically reach out to recipients to request information for the completion of desk reviews. Requested information may include:
Policies and procedures
List of grant expenditures
Accounting records
Supporting documents (e.g., invoices, receipts, paystubs, timesheets, contracts, etc.)
Financial statements
Audit reports
Other related documentation
2. Site visits: FDA will conduct site visits when necessary and will notify the recipient with reasonable advance notice of any such visit(s).
3. Foreign entities: All Foreign entities are subject to the same monitoring requirements as domestic entities. Foreign entities covered under immunity Executive Orders will provide supporting documents for monitoring requirements unless such an action is a violation of the Executive Orders. Recipients may discuss with the FDA to come up with an alternate approach to satisfy the award monitoring requirements.
All recipients will make reasonable efforts to resolve issues found, including audit findings. Successful resolutions to issues are important as they are part of the grant performance review. All recipients are responsible for submitting all requested information in an expeditious manner. Failure to submit timely reports and/or respond to inquiries from FDA may affect future funding or enforcement actions, including withholding, or conversion to a reimbursement payment method.
Financial Conflict of Interest (FCOI):
This award is subject to the Financial Conflict of Interest (FCOI) regulation at 42 CFR Part 50 Subpart F.
Closeout Requirements (when applicable):
A Final Research Performance Progress Report (FRPPR), Final Federal Financial Report SF-425, Final Invention Statement HHS-568 (if applicable), Tangible Personal Property Report SF-428 (if applicable), and Statement of Disposition of Equipment (if applicable) must be submitted within 90 days after the expiration date of the project period. All closeout documents must be submitted electronically in eRA Commons.
The Final FFR must indicate the exact balance of unobligated funds and may not reflect unliquidated obligations. There must be no discrepancies between the Final FFR expenditure data and FFR cash transaction data in the Payment Management System (PMS). The expended funds reported on the Final FFR must exactly match the disbursements reported on the grantee's report to the Payment Management System and the charge advances in PMS. It is the recipient's responsibility to reconcile reports submitted to PMS and to the FDA.
Program Income:
The grantee is required to report any Program Income generated during the Project Period of this grant. Except for royalty income generated from patents and inventions, the amount and disposition of Program Income must be identified on lines 10 (l), (m), (n), and (o) of the grantee s Federal Financial Report (FFR) SF-425.
Examples of Program Income include (but are not limited to): fees for services performed during the grant or sub-grant period, proceeds from sale of tangible personal or real property, usage or rental fees, patent or copyright royalties, and proceeds from the sale of products and technology developed under the grant.
Any Program Income generated during the Project Period of this grant by the grantee or sub-grantee will be treated as identified below.
Treatment of Program Income:
Prohibition on certain telecommunications and video surveillance services or equipment:
(a) As described in CFR 200.216, recipients and subrecipients are prohibited to obligate or spend grant funds (to include direct and indirect expenditures as well as cost share and program) to:
(1) Procure or obtain,
(2) Extend or renew a contract to procure or obtain; or
(3) Enter into contract (or extend or renew contract) to procure or obtain equipment, services, or systems that use covered telecommunications equipment or services as a substantial or essential component of any system, or as critical technology as part of any system. As described in Pub. L. 115-232, section 889, covered telecommunications equipment is telecommunications equipment produced by Huawei Technologies Company or ZTE Corporation (or any subsidiary or affiliate of such entities).
i. For the purpose of public safety, security of government facilities, physical security surveillance of critical infrastructure, and other national security purposes, video surveillance and telecommunications equipment produced by Hytera Communications Corporation, Hangzhou Hikvision Digital Technology Company, or Dahua Technology Company (or any subsidiary or affiliate of such entities).
ii. Telecommunications or video surveillance services provided by such entities or using such equipment.
iii. Telecommunications or video surveillance equipment or services produced or provided by an entity that the Secretary of Defense, in consultation with the Director of the National Intelligence or the Director of the Federal Bureau of Investigation, reasonably believes to be an entity owned or controlled by, or otherwise, connected to the government of a covered foreign country.
Other:
This award is subject to the requirements of 2 CFR Part 25 for institutions to maintain an active registration in the System of Award Management (SAM). Should a consortium/subaward be issued under this award, a requirement for active registration in SAM must be included.
In accordance with the regulatory requirements provided at 45 CFR 75.113 and Appendix XII to 45 CFR Part 75, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts with cumulative total value greater than $10,000,000 must report and maintain information in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (currently the Federal Awardee Performance and Integrity Information System (FAPIIS)). Full reporting requirements and procedures are found in Appendix XII to 45 CFR Part 75.
Other:
A majority of the cooperative agreement funds should be spent to build relationships and assist SLTT regulatory agencies with primary responsibility for retail food establishments in their jurisdictions or to agencies with substantial control and responsibility over such agencies, which may include financial support.
Additional Reporting Requirements:
All FDA grants require annual financial and performance progress reporting. This award has additional performance reporting requirements as outlined below.
Mid-Year interim performance progress reports are required for this award. The interim performance progress reports should be submitted via email to the listed Grants Management Specialist and Program Official by October 31, for each budget period.
Mid-year and annual progress reports shall contain the elements below as applicable to the grantee proposal and award, but are not limited to, the following:
1. Detailed progress report on the grantee meeting the project milestones, objectives, and defined performance outcomes requested by FDA. If other associations or organizations are utilized to achieve project milestones or objectives, the progress report should include their activities.
2. Summary of funding requests received, and financial assistance issued with the following information, at a minimum: name and contact information of agency, summary project proposed, funds requested, funding decision, and progress of the projects selected for funding.
3. Status report on the hiring and training of any personnel.
4. Status report on the purchasing and operational readiness of any equipment, computers, or software purchased.
5. Identify any pending issues or concerns that may affect accomplishing the objectives and goals of the cooperative agreement, including any sub-awards made. If the objectives and goals of the cooperative agreement are not being met, then a corrective action plan shall be submitted. The corrective action plan should detail the tasks, responsible personnel, and updated timeframes to ensure satisfactory performance and meet the deliverables required under the grant.
6. Detailed program budget demonstrating that a majority of the cooperative agreement funds are being spent to build relationships and assist SLTT regulatory agencies with primary responsibility for retail food establishments in their jurisdictions or to agencies with substantial control and responsibility over such agencies
Cooperative Agreement Terms and Conditions of Award
The following special terms of award are in addition to, and not in lieu of, otherwise applicable U.S. Office of Management and Budget (OMB) administrative guidelines, U.S. Department of Health and Human Services (DHHS) grant administration regulations at 45 CFR Part 75, and other HHS, PHS, and FDA grant administration policies.
The administrative and funding instrument used for this program will be the cooperative agreement, an assistance mechanism (rather than an acquisition mechanism), in which substantial FDA programmatic involvement with the awardees is anticipated during the performance of the activities. Under the cooperative agreement, FDA’s objective is to support and stimulate the recipient’s activities by involvement in and otherwise working jointly with the award recipient in a partnership role; it is not to assume direction, prime responsibility, or a dominant role of activities. Consistent with this concept, the dominant role and prime responsibility resides with the awardee for the project as a whole, although specific tasks and activities may be shared between the awardee and the FDA as defined below.
Substantive involvement includes, but is not limited to, the following:
- FDA review and approval of task-oriented guidelines and other documents developed under the award.
- FDA assistance and coordination in the sharing of information to Federal, State, and local agencies and other stakeholders.
- FDA review and approval of publications/web applications or any revisions to existing systems.
- FDA review and approval of financial assistance made using cooperative agreement funds. FDA will coordinate with the grantee on final subaward decisions, and recommend changes to funding amount, priority, and other aspects to ensure the subaward meets the FDA’s intended goals.
- Other assistance or collaboration as requested by associations and State/local agencies.
- FDA will have a minimum of four (4) meetings per year to review grantee progress and discuss cooperative agreement goals and objectives.
The program project manager, program official, grants management officer, technical advisor, FDA Retail Food Specialist, and representatives from other components within FDA will monitor the recipient and sub-awardees. The monitoring may be in the form of telephone conversations, e-mails, or written correspondence between the FDA and the principal investigator. Periodic site visits from representatives of ORA with officials of the recipient organization or sub-awardees may also occur. There may be other regular meetings with the recipient and sub-awardees to assist in fulfilling the requirements of the cooperative agreement.
Principal Investigator Rights and Responsibilities
The Principal Investigator will have the primary responsibility for and dominant role in planning, directing, and executing the proposed project, with the FDA staff being substantially involved as a partner with the PI.
Awardees will retain custody of and have primary rights to the data and software developed under these awards, subject to Government rights of access consistent with current HHS, PHS, and FDA policies.
FDA Responsibilities
a. FDA will have prior approval of the appointment of all key administrative and scientific personnel proposed by the grantee.
b. FDA scientists and subject matter experts will participate with the grantee, in determining and carrying out scientific and technical activities. Collaboration will also include data analysis, interpretation of findings and, where appropriate, co-authorship of publications.
In addition, work proposed and conducted under this cooperative agreement, including subawards issued, may not be duplicated or funded by other cooperative agreements, contracts, or other funding mechanisms. Projects proposed and conducted under this cooperative agreement and the funding provided shall remain distinct and separate from other projects and funding sources. The grantee shall be able to account separately for fund expenditures, including employee salaries, wages, and benefits, received through contracts, cooperative agreements, grants, and other funding received by the grantee and these cooperative agreements.
When multiple years are involved, awardees will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the Notice of Award.
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the terms and conditions of award and the HHS Grants Policy Statement.
The Federal Funding Accountability and Transparency Act of 2006 (Transparency Act), includes a requirement for awardees of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All awardees of applicable FDA grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over $25,000.
In accordance with the regulatory requirements provided at 45 CFR 75.113 and Appendix XII to 45 CFR Part 75, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (currently FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 45 CFR Part 75 Award Term and Conditions for Recipient Integrity and Performance Matters.
We encourage inquiries concerning this funding opportunity
and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten submission by the due date, and post-submission issues)
Finding Help Online: http://grants.nih.gov/support/ (preferred
method of contact)
Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
Grants.gov Customer Support (Questions regarding
Grants.gov registration and Workspace)
Contact Center Telephone: 800-518-4726
Email: support@grants.gov
Suzanne Webb
Office of Management (OM)
Food and Drug Administration
Telephone: 240-402-3069
Email: Suzanne.Webb@fda.hhs.gov
Maribeth Niesen
Office of Regulatory Affairs (ORA)
Telephone: 513-679-2704
Email: maribeth.niesen@fda.hhs.gov
Gordana Zuber
Office of Acquisitions & Grants Services (OAGS)
Food and Drug Administration
Telephone: 301-348-1747
Email: gordana.zuber@fda.hhs.gov
Gordana Zuber
Office of Acquisitions & Grants Services (OAGS)
Food and Drug Administration
Telephone: 301-348-1747
Email: gordana.zuber@fda.hhs.gov
All awards are subject to the terms and conditions, cost principles, and other considerations described in the HHS Grants Policy Statement, 45 CFR 75 and Notice of Award.
Awards are made under the authorization of section 1009 of the Federal Food, Drug, and Cosmetic Act (21 USC 399) and under Federal Regulations 42 CFR Part 52 and 45 CFR Part 75.

